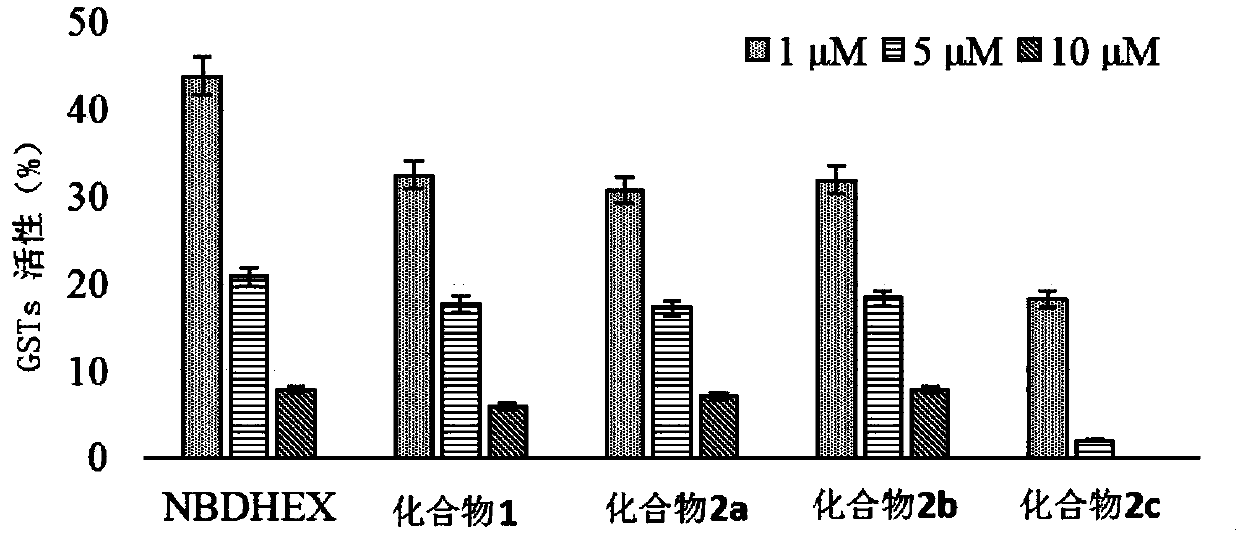
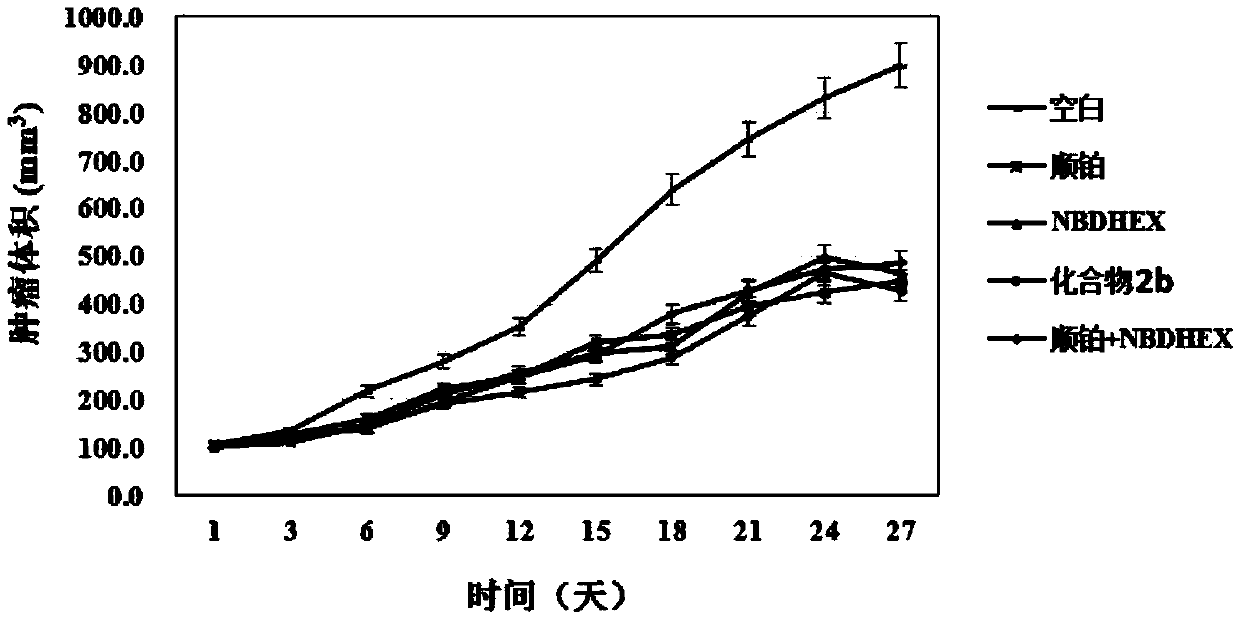
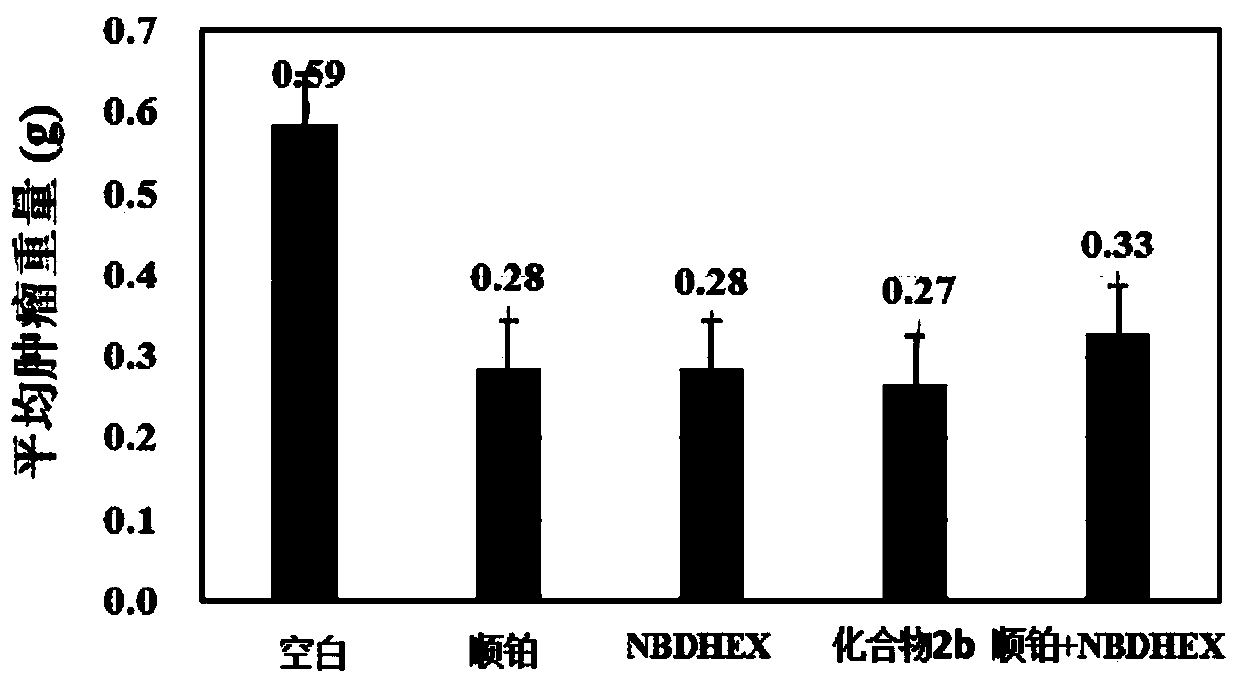
A compound capable of suppressing glutathione S-transferase and a preparing method thereof
A technology of sulfhydryl transferase and glutathione, which is applied in the field of anti-tumor drugs, can solve the problems of toxicity and side effects that limit clinical application, and achieve the effects of overcoming cisplatin resistance, low toxicity and side effects, and significant anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound 1
[0042] Weigh 1.15g NBDHEX, 1.16g succinic anhydride and 1.42g DMAP in a 100ml round bottom flask, add 40ml dichloromethane, heat and reflux for 6 hours, after TLC detects that the NBDHEX reaction is complete, then wash once with 1M hydrochloric acid solution, Washed once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and purified by silica gel column chromatography, and gradient eluted with a mixed solvent of dichloromethane and methanol to obtain 1.33 g of the product with a yield of 86.3%.
[0043] ESI-MS: [M-H] - =396.08. 1 H-NMR (ppm, 300MHz, CDCl 3 ): δ8.43(d, J=9.0Hz, 1H), δ7.18(d, J=6.0Hz, 1H), δ4.14(t, J=7.5Hz, 2H), δ3.30(t, J=7.5Hz, 2H), δ2.67(4H), δ1.95~1.82(m, 2H), δ1.75~1.67(m, 2H), δ1.67~1.53(m, 2H), δ1. 53~1.42(m,2H).
Embodiment 2
[0044] Embodiment 2: the preparation of compound 2a
[0045] Weigh 218.6mg of Compound 1 and 176.0mg of TBTU into a 25ml round bottom flask, add 7ml of DMF, and stir at 40°C for 15min. Measure 85 μl of TEA and add it, and continue to stir for 15 minutes. Then weighed 176.3 mg of tetravalent platinum complex A and added it, raised the temperature to 50°C, and stirred in the dark for 24 hours, distilled off the solvent under reduced pressure, purified by silica gel column chromatography, and carried out gradient washing with a mixed solvent of petroleum ether and ethyl acetate. After removal, 110.0 mg of the product was obtained, with a yield of 30.1%.
[0046] ESI-MS: [M-H] - =730.0734. 1 H-NMR (ppm, 300MHz, DMSO-d 6 ): δ8.55(d, J=6.0Hz, 1H), δ7.50(d, J=6.0Hz, 1H), δ6.12(-NH 3 ,6H),δ4.00(t,J=7.5Hz,2H),δ3.52(t,J=7.5Hz,2H),δ2.60~2.30(m,4H),δ1.83~1.70(m ,2H), δ1.65~1.30(m,6H). 13 C-NMR (ppm, 75MHz, DMSO-d 6 ): δ173.87, 172.60, 149.65, 143.16, 140.45, 132.82, 132.57, 122.62...
Embodiment 3
[0047] Embodiment 3: the preparation of compound 2b
[0048] Weigh 198.7mg of compound 1 and 176.0mg of TBTU into a 25ml round bottom flask, add 7ml of DMF, and stir at 40°C for 15min. Measure 85 μl of TEA and add it, and continue to stir for 15 minutes. Then weighed 167.0 mg of tetravalent platinum complex B and added it, raised the temperature to 50°C, and stirred in the dark for 24 hours, distilled off the solvent under reduced pressure, purified by silica gel column chromatography, and carried out gradient washing with a mixed solvent of petroleum ether and ethyl acetate. 75.0 mg of the product was obtained with a yield of 21.0%.
[0049] ESI-MS: [M-H] - =712.0870. 1 H-NMR (ppm, 500MHz, DMSO-d 6 ): δ8.60(d, J=10.0Hz, 1H), δ7.55(d, J=10.0Hz, 1H), δ5.93(-NH 3 ,6H),δ4.04(t,J=7.5Hz,2H),δ3.40(t,J=7.5Hz,2H),δ2.47(s,4H),δ1.85~1.77(m,2H ), δ1.68~1.57(m,2H), δ1.56~1.47(m,2H), δ1.45~1.37(m,2H). 13 C-NMR (ppm, 125MHz, DMSO-d 6 ): δ173.27, 172.03, 149.10, 142.58, 139.95, 132.2...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap