A kind of carbazole derivative mcab and its preparation method and application
A carbazole derivative, carbazole technology, applied in chemical instruments and methods, fluorescence/phosphorescence, instruments, etc., can solve the problems of cytotoxicity, limited fluorescent probes, and no lysosome targeting, and achieve cost The effect of low cost, simple detection method and good cell membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation and Characterization of 1,4-(3-(9-(2-(2-methoxyethoxy)ethyl-9H-carbazol-3-yl)-3-acryloyl)benzaldehyde (MCAB) :
[0025]
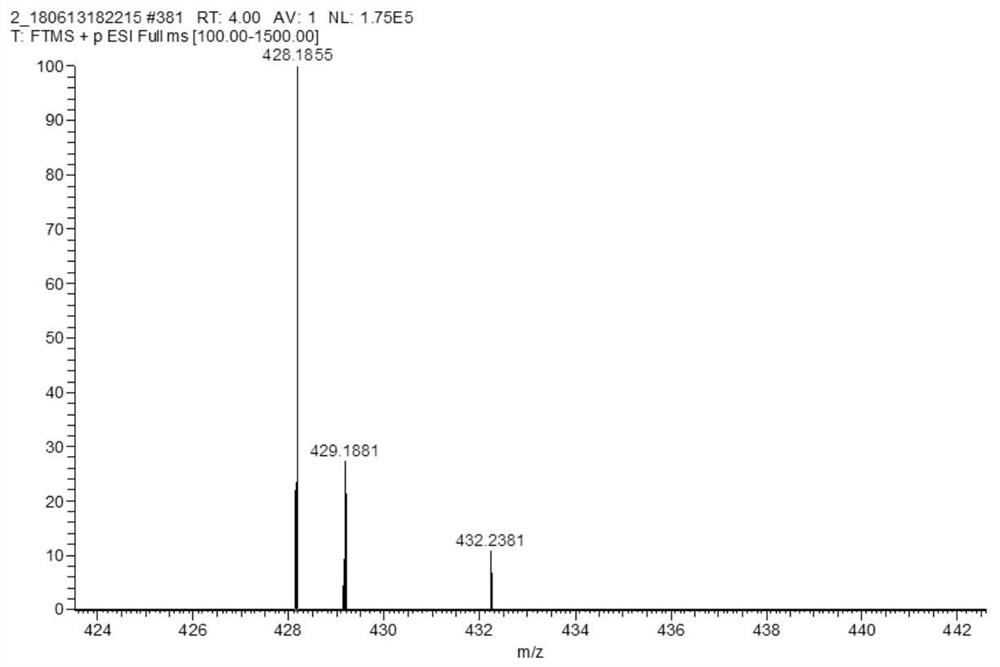
[0026] In a three-necked flask, mix 1-(9-(2-(2-methoxyethoxy)ethyl)-9H-carbazol-3-yl)ethanone (70mg, 0.22mmol) and terephthalaldehyde (45mg, 0.33mmol) was dissolved in ethanol, added 3-5 drops of piperidine, and heated to reflux for 12h until the reaction was complete; the system was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product; the crude product was purified by silica gel column (eluent According to the volume ratio of chloroform:methanol=20:1), the dark green solid was obtained as the target compound MCAB (probe).
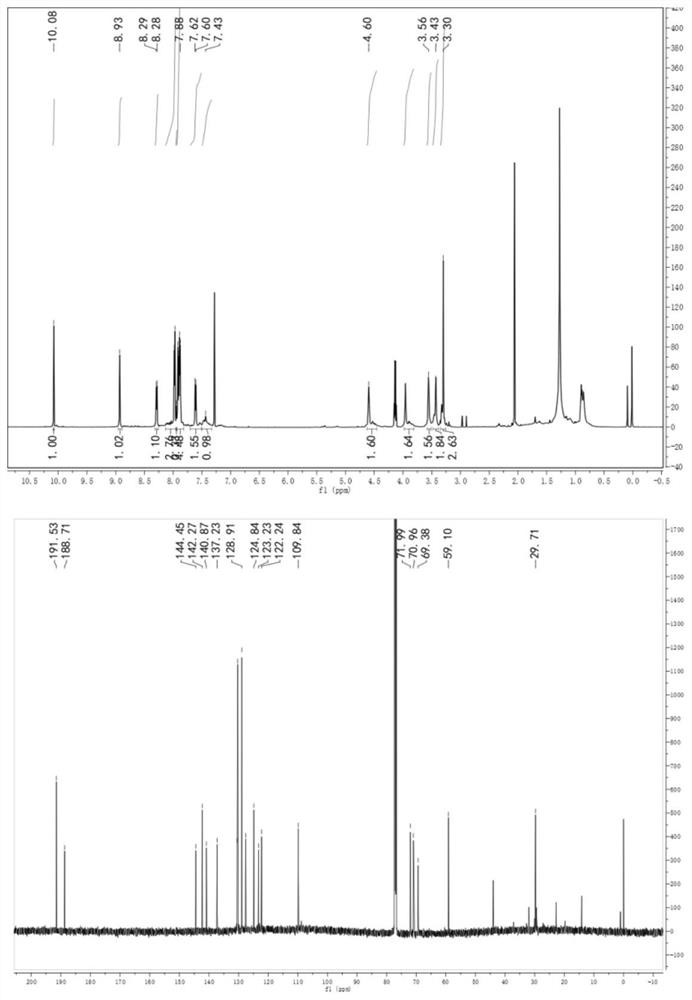
[0027] 1 H NMR (600MHz, CDCl 3 , figure 1 Middle and upper picture) δ(ppm): 3.30(3H), 3.43(2H), 3.56(2H), 3.96(2H), 4.60(2H), 7.40-7.43(1H), 7.59-7.62(2H), 7.85- 8.00(9H), 8.24-8.28(1H), 8.93(1H).
[0028] 13 C NMR (150MHz, CDCl 3 , ...
Embodiment 2
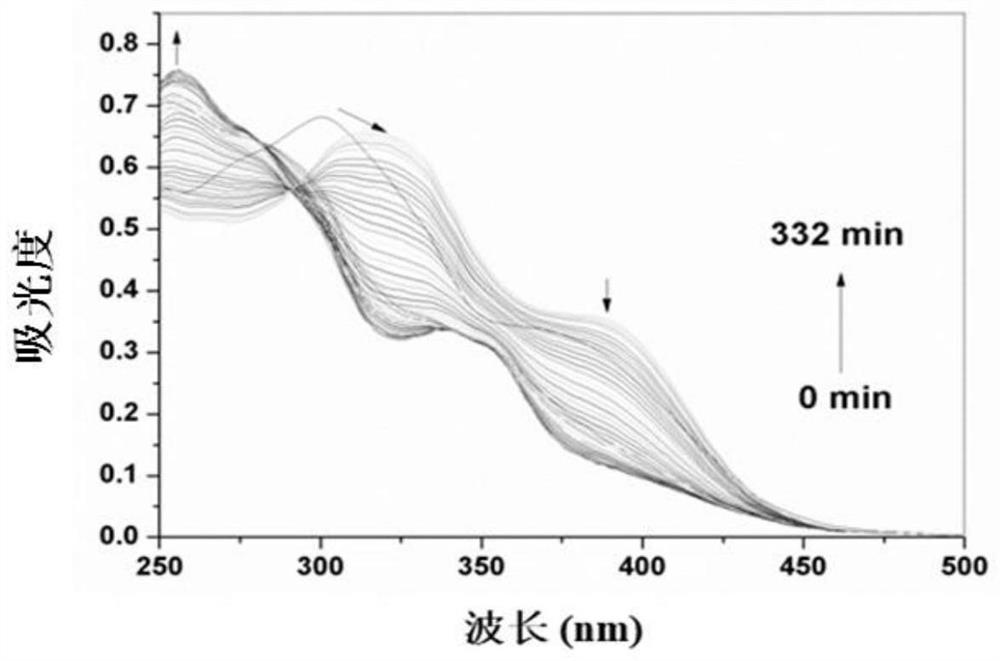
[0031] The probe MCAB was diluted to 25 μM with DMSO / PBS buffer (pH 7.4) system (v / v=1 / 1), and the ultraviolet absorption spectrum of the reaction between MCAB and Cys (500 μM) changed with time was recorded. Such as image 3 As shown, after adding Cys, the UV absorbance at 300 nm and 388 nm changed to 255 nm and 338 nm.
Embodiment 3
[0033] Dilute the probe MCAB to 25 μM with DMSO / PBS buffer (pH 7.4) system (v / v=1 / 1), record the fluorescence spectrum of the reaction between MCAB and Cys (500 μM) over time, fix the excitation wavelength at 370 nm, and and emission slit broadband are both 2.0nm. After adding Cys, a new emission peak appeared at 440nm and increased sequentially with time. The fluorescence intensity reached the maximum value ( Figure 4 ). Under the irradiation of ultraviolet light, the color of the solution changed from colorless to blue ( Figure 5 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| UV absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com