Antibacterial property test method of wet wipe products
A test method, technology of wet wipes, applied in the field of health care, can solve problems such as the inability to investigate antibacterial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] 1. Preparation of bacterial suspension
[0028] Activate Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa on the TSA plate for 18-24 hours respectively, at a culture temperature of 30-35°C;
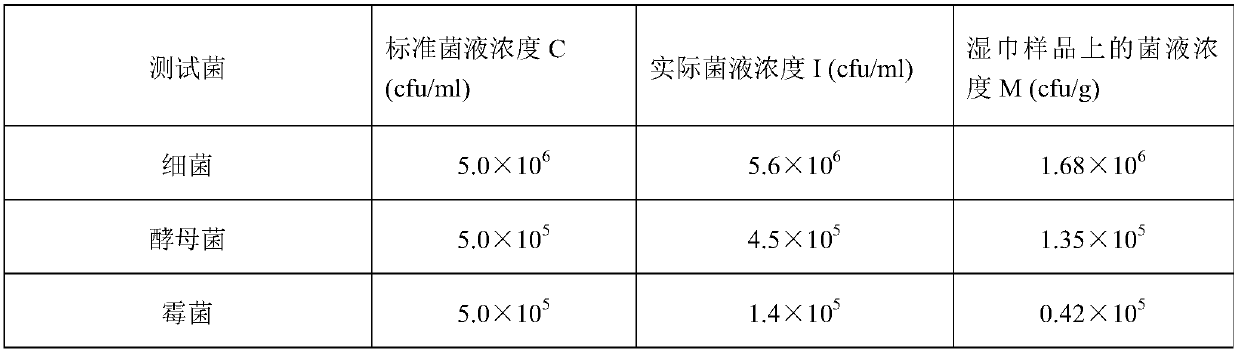
[0029] Then use 0.85% sterile normal saline to elute the above three strains respectively, and prepare 5.0×10 6 cfu / ml bacterial suspension;
[0030] Finally, all bacterial suspensions were mixed to prepare 5.0×10 6 cfu / ml mixed bacterial suspension.
[0031] 2. Preparation of yeast suspension
[0032] Cultivate Candida albicans on SDA medium for 48 hours at a temperature of 20-25°C;
[0033] Then the Candida albicans strains were eluted with 0.85% sterile saline, and prepared into 5.0×10 5 cfu / ml bacterial suspension.
[0034] 3. Preparation of mold suspension
[0035] Cultivate Aspergillus niger species on SDA medium for 7 days at a culture temperature of 20-25°C;
[0036] Then with 0.85% sterile normal saline plus Tween 80 with a volume concentration of...
Embodiment 1
[0072] In Example 1, the wet tissue sample used was 5g / piece*3 pieces / pack, and the volume Vi of the inoculated bacterial suspension on each wet tissue sample=3×5g×0.3ml / g=4.5ml;
[0073] The volume of diluent added E = 5g x 3 x 10ml / g - 5g x 3 x 1ml / g - 4.5ml = 130.5ml.
[0074] Table 1 is the parameter of three test bacterial suspensions of embodiment 1.
[0075] Table 1
[0076]
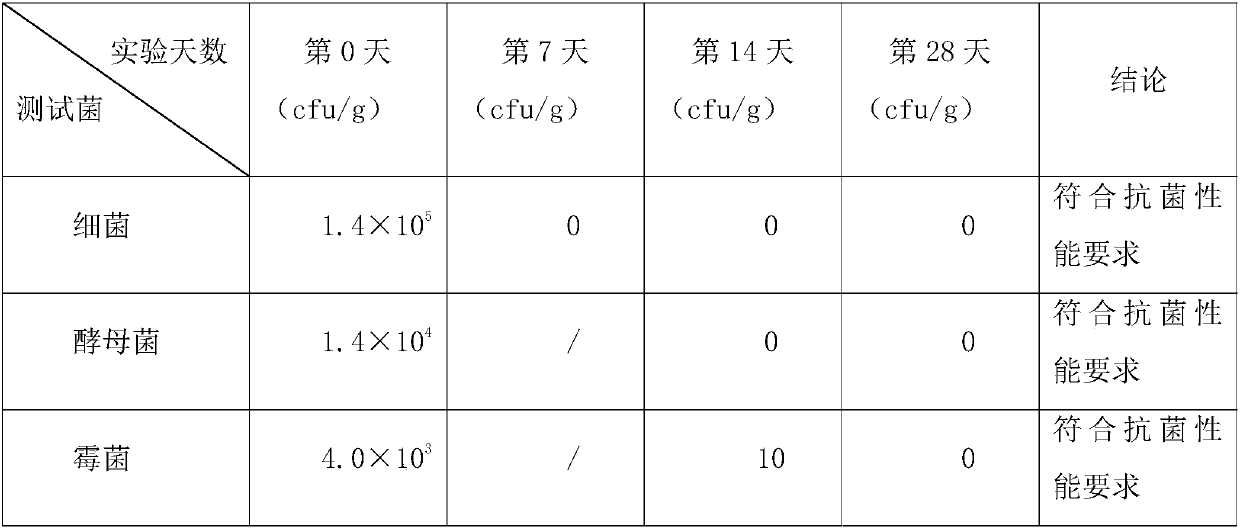
[0077] Table 2 shows the number of colonies of the wet tissue samples of Example 1 at various time points during the test.
[0078] Table 2
[0079]
[0080] It can be seen from Table 2 that the number of colonies of bacteria on and after the 7th day was 0, reducing by 5 log levels, meeting the antibacterial performance requirements; the number of colonies of yeast on and after the 14th day was 0, reducing by 4 log levels, It meets the antibacterial performance requirements; after the 14th day, the number of colonies of mold is 10, a reduction of 2 log levels, which meets the antibacteria...
Embodiment 2
[0082] In Example 2, the wet tissue sample used is 16.5g / piece*1 piece / pack, and the volume Vi of the inoculated bacterial suspension on each wet tissue sample=16.5g×0.3ml / g=4.95ml;
[0083] The volume of diluent added E = 16.5g x 10ml / g - 16.5g x 1ml / g - 4.95ml = 143.55ml.
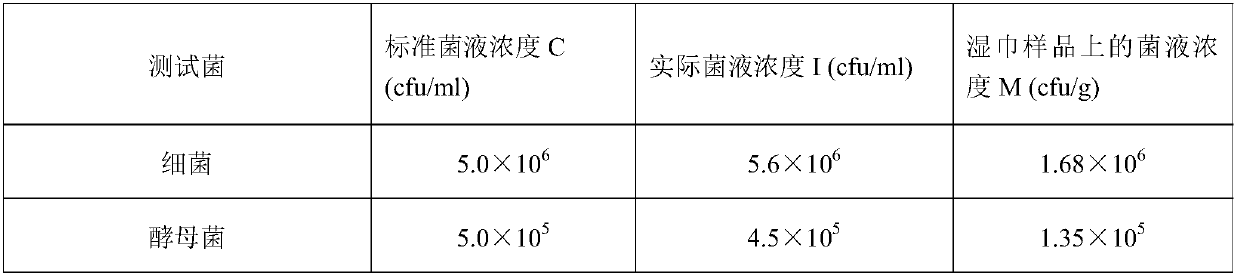
[0084] Table 3 is the parameter of three test bacterial suspensions of embodiment 2.
[0085] table 3
[0086]
[0087]
[0088] Table 4 shows the number of colonies of the wet tissue samples of Example 2 at various time points during the test.
[0089] Table 4
[0090]
[0091] It can be seen from Table 4 that the number of colonies of bacteria on and after the 7th day was 0, reducing by 5 log levels, meeting the antibacterial performance requirements; the number of colonies of yeast on and after the 14th day was 0, reducing by 4 log levels, It meets the antibacterial performance requirements; the number of mold colonies after the 14th day is 8, a reduction of 2 log levels, which meets the an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com