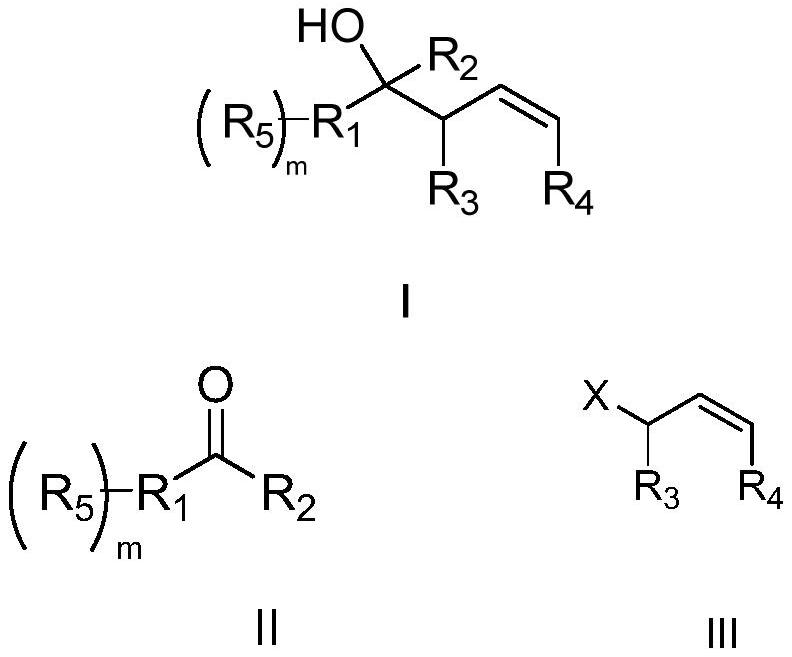
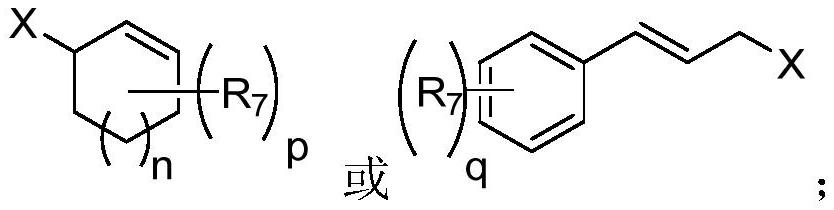
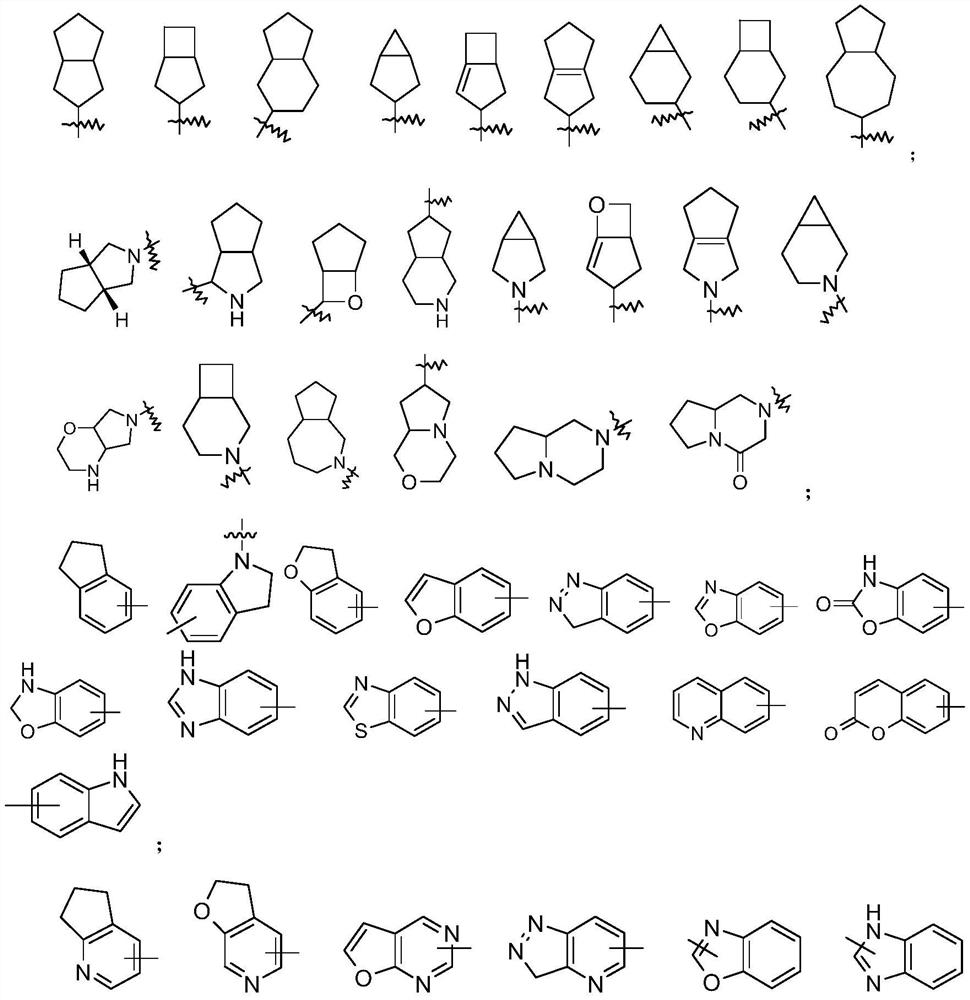
A kind of preparation method of homoallyl alcohol compound
A compound and alkyl technology, applied in the field of preparation of homoallyl alcohol compounds, can solve problems such as limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]
[0067] Iron powder (83.8 mg, 1.5 mmol) and DMSO (1 mL) were added sequentially to a 10 mL Schlenk bottle. The iron was then activated by adding 1,2-dibromoethane (14.1 mg, 5 mol%) and TMSCl (8.1 mg, 5 mol%). With stirring, BiCl was sequentially added to the reaction mixture 3 (31.5 mg, 0.1 mmol), 2a (241.5 mg, 1.5 mmol) and 1a (82 mg, 0.5 mmol). The suspension was stirred vigorously at room temperature for 24 h, then washed with saturated NaHCO 3 The aqueous solution was quenched. use NH 4 Cl solution (30 mL) was washed, and extracted with ethyl acetate (20 mL×3). The combined extracts were washed with brine (30 mL), dried over anhydrous sodium sulfate and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography using ethyl acetate / petroleum ether as eluent to obtain 118.08 mg of 3a with a yield of 96% and a dr value >99:1.
Embodiment 2
[0069] According to the method of Example 1, the catalysts in the table were used to prepare 3a.
[0070]
Embodiment 3
[0072] 3a was prepared according to the method of Example 1 using the metals in the table.
[0073] Metal Yield (%) Mg 32 Al <5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com