Process for preparing benzofuranone derivatives
A compound, the technology of trimethyl orthoformate, applied in the field of intermediates in the method of preparing phenoxypyrimidine compounds, can solve problems such as failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
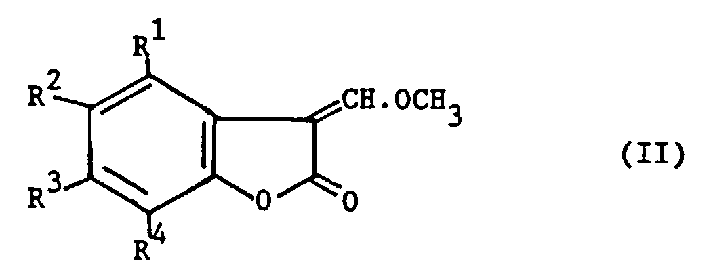
[0182] This example illustrates the preparation method of 3-(α-methoxy)methylenebenzofuran-2(3H)one.
[0183] Benzofuran-2(3H)one (10.2g), acetic anhydride (30ml) and trimethyl orthoformate (112.1g) were stirred at 100-105°C for 12 hours. During this period, a Dean-Stark device was used to collect low-boiling liquids.
[0184] The reaction mixture was allowed to cool and concentrated under reduced pressure (using a water bath temperature of 60°C) to obtain a brown solid. This solid was dissolved in dichloromethane (100ml), the solution was washed with water (2×50ml) and concentrated under reduced pressure (using a water bath temperature of 60°C) to obtain a crude product (13.5g). A part of the crude product was added to the crude product obtained from similar experiments, and the entire crude product was dissolved in methanol, and then treated with activated carbon. Thereafter, the methanol solution was refluxed for 30 minutes, cooled to below 10°C, filtered and the residue was wa...
example 2
[0188] This example illustrates another preparation method of 3-(α-methoxy)methylenebenzofuran-2(3H)one.
[0189] Ortho-hydroxyphenylacetic acid (15.2g), toluene (95ml) and glacial acetic acid (5ml) were mixed and heated to reflux for 4 hours, after which there was no undissolved raw material. During this period, water (2.2ml) was collected using a Dean-Stark device. Then, the reaction mixture was cooled and allowed to stand overnight.
[0190] Then acetic anhydride (40ml) was added to the reaction mixture and the low boiling point solvent (mostly toluene) (100ml) was distilled off. After cooling to below 50°C, trimethyl orthoformate (15.9 g) was added to the reaction mixture, and then the mixture was heated to 100-105°C for 20 hours. Gas chromatographic analysis showed that about 5% of the raw material remained.
[0191] The reaction mixture was worked up and purified as described in Example 1.
example 3
[0193] This example illustrates another preparation method of 3-(α-methoxy)methylenebenzofuran-2(3H)one.
[0194] Benzofuran-2(3H)one (10g), o-hydroxyphenylacetic acid (11.3g), acetic anhydride (60ml) and trimethyl orthoformate (23.7g) were heated to 100-105°C for 14 hours. During this period, a Dean-Stark device was used to collect some volatile products. Analysis of the reaction mixture indicated that about 5% of the starting material was still present.
[0195] The reaction mixture was concentrated under reduced pressure (water bath at 70°C) to obtain a crude product (28-24 g). This was combined with the crude product obtained from similar experiments and recrystallized from methanol to obtain the title compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com