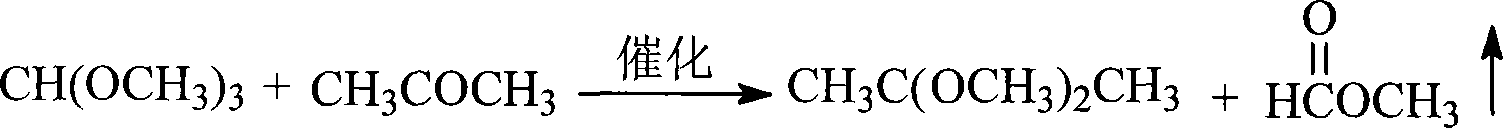Method for synthesizing 2,2-dimethoxypropane
A technology of dimethoxypropane and a synthesis method, which is applied in 2 fields, can solve the problems of complex process, reduced yield, strong corrosion of equipment, etc., and achieves the effects of simple equipment requirements, easy scale and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take a reactor with a rectification device, add 20g of p-toluenesulfonic acid catalyst and 500mL of acetone in the reactor, start stirring, add 825mL of trimethyl orthoformate dropwise, control the temperature at 30-40°C, and collect methyl formate at the top of the tower , reacted for 3 to 4 hours, after the reaction solution was neutralized by adding sodium carbonate, rectification under reduced pressure (30mmHg), keep the temperature in the reactor at 30 to 35°C, circulate the refrigerated medium to cool the fraction, collect DMP, the product content > 95%, Yield 85~92%.
Embodiment 2
[0020] Take a reactor with a rectification device, add 30g of catalyst potassium bisulfite and 700mL of acetone into the reactor, start stirring, add 120mL of trimethyl orthoformate dropwise, control the temperature of the reactor at 10-15°C, and control the pressure (50mmHg ), cooling and collecting methyl formate by circulating refrigeration medium at the top of the tower, reacting for 7 to 8 hours, filtering out the catalyst, adding sodium carbonate to the filtrate to neutralize, rectifying under reduced pressure (25mHg), keeping the temperature in the reactor at 25 to 30°C, and circulating Refrigeration medium cools the distillate, collects the medium boiler and the product DMP, the medium boiler can be applied mechanically, the product DMP content is > 95%, and the yield is 85-90%.
Embodiment 3
[0022] Take the same device as in Example 1, and the same operation, add 1000 mL of trimethyl orthoformate dropwise to 15 g of catalyst ammonium chloride and 75 mL of acetone, control the temperature of the reactor at 30 to 40 ° C, collect methyl formate at the top of the tower, and react After 3-4 hours, filter the catalyst, neutralize the filtrate with sodium carbonate, rectify under reduced pressure (40mmHg), keep the temperature in the reactor at 35-40°C, cool with circulating refrigeration medium, collect the product DMP, and recycle the substrate. Product DMP content > 95%, yield 85-92%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com