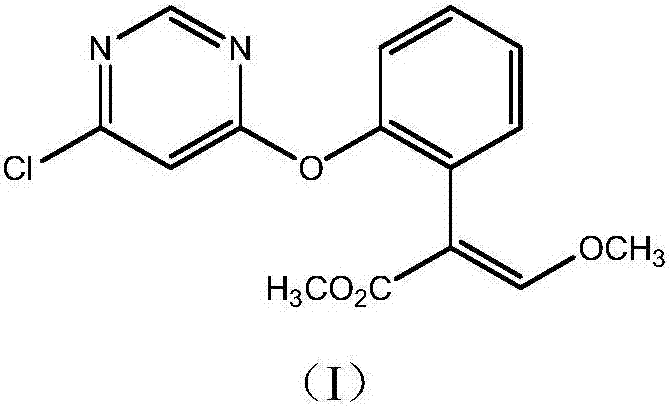
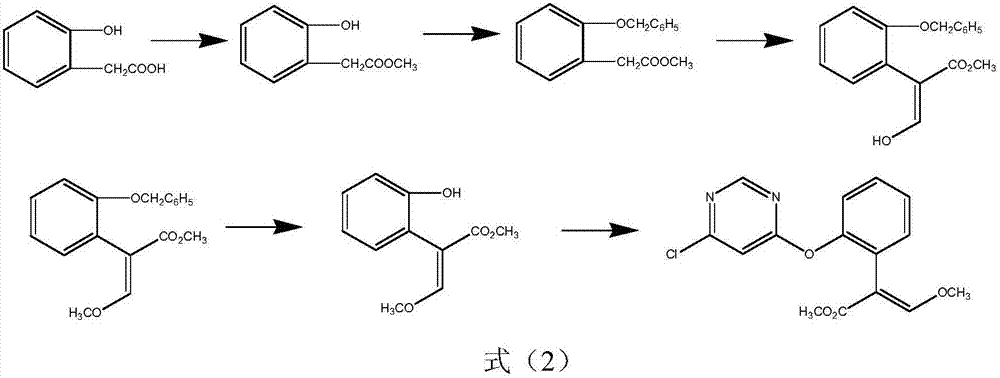
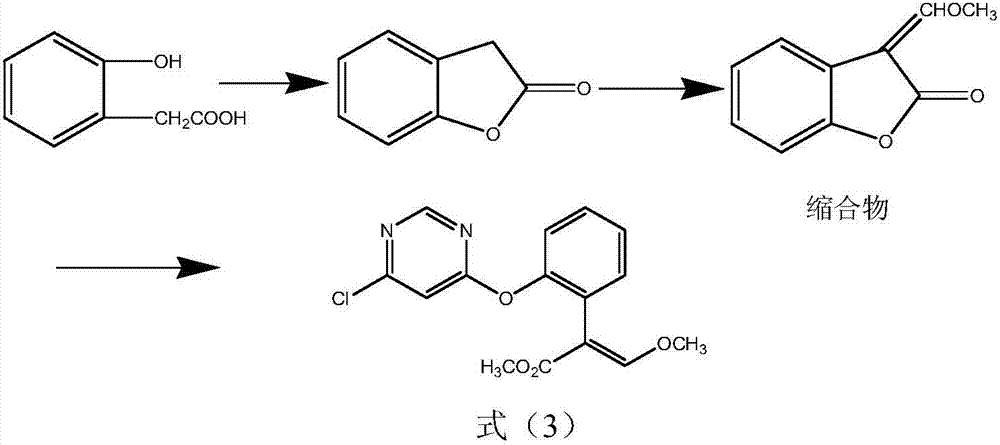
Synthetic method for azoxystrobin intermediate
A synthesis method and intermediate technology, which are applied in the synthesis field of azoxystrobin intermediates, can solve the problems of complicated operation, low yield, many by-products, etc., and achieve the advantages of lowering reaction temperature, improving yield and reducing loss of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add benzofuranone 400g (2.98mol) trimethyl orthoformate 600g (5.66mol) and acetic anhydride 670ml in the four-necked flask, and add a rectifying column with a length of 20cm on the bottleneck, and the filler is a glass spring. Distillation reaction is carried out, the temperature in the reaction bottle is slowly raised to 95-100°C, the temperature is raised for about 2-3h, and the temperature is kept for about 10h. During the reaction process, the low-boiling methyl acetate produced by the reaction is distilled through the rectification column to reduce the entrainment of raw materials such as trimethyl orthoformate and acetic anhydride, and it is slightly cooled and distilled under reduced pressure to 100°C. 1h, add toluene and stir to dissolve, and directly use the next step without treatment (quantitative yield 96.5%).
[0025] Add 488g of 2,6-dichloropyrimidine to a four-neck flask, add 900ml of toluene, add the condensate toluene solution from the previous step, ad...
Embodiment 2
[0027] Add benzofuranone 400g (2.98mol) trimethyl orthoformate 505g (4.76mol) and acetic anhydride 600ml in the four-necked flask, and add a rectifying column with a length of 40cm on the bottleneck, the packing is a ceramic packing, Distillation reaction is carried out, the temperature in the reaction bottle is slowly raised to 95-100°C, the temperature is raised for about 2-3h, and the temperature is kept for about 10h. During the reaction process, the low-boiling methyl acetate produced by the reaction is distilled through the rectification column to reduce the entrainment of raw materials such as trimethyl orthoformate and acetic anhydride, and it is slightly cooled and distilled under reduced pressure to 100°C. 1h, add toluene and stir to dissolve, and directly use the next step without treatment (quantitative yield 93.3%).
[0028] Add 488g of 2,6-dichloropyrimidine to the four-neck flask, add 900ml of toluene, add the condensate toluene solution from the previous step, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com