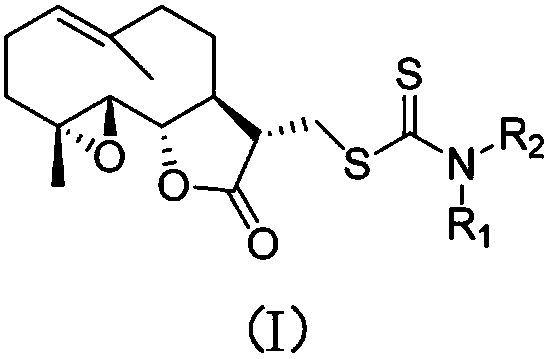
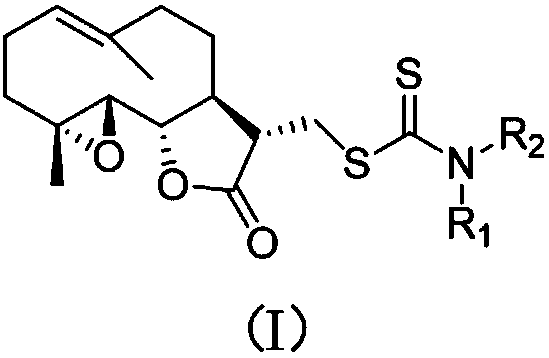
Parthenolide dithiocarbamate derivative, salt and medicinal composition thereof, and use of derivative, salt or medicinal composition
A technology of ester dithiocarbamate and derivatives is applied in the application field of preparing anti-cancer or auxiliary anti-cancer drugs, and can solve the problems of water solubility, poor plasma stability, low activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
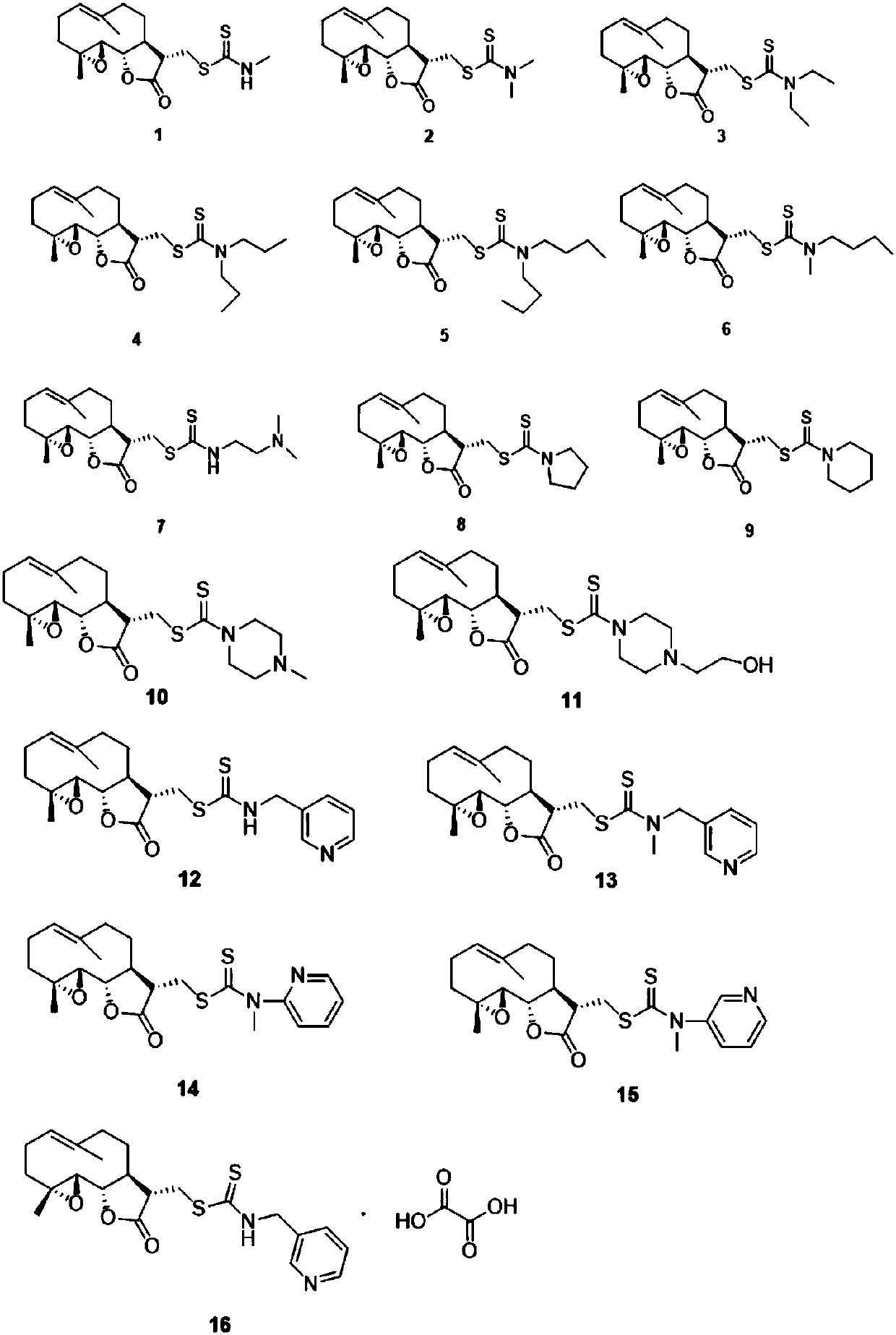
[0014] Example 1: Synthesis of compound 1-16
[0015] The nucleophilic addition reaction of various amines with carbon disulfide under the action of triethylamine produces various dithiocarbamates, and then performs Michael addition with the α, β-unsaturated double bond of parthenolide Reaction, one-pot method to prepare 1-13. After the aminopyridine is reacted with butyl lithium, it undergoes nucleophilic addition reaction with carbon disulfide to prepare dithioformate, and then undergoes Michael addition reaction with parthenolide to obtain compound 14 or 15.
[0016] Synthesis of compound 1
[0017] Place the reaction vial in an ice-water bath, add the solvents dichloromethane (2mL) and methanol (0.5mL), carbon disulfide (0.1mL, 1.5mmol) and triethylamine (0.15mL, 1.1mmol) in sequence, start stirring, Add methylamine hydrochloride (81mg, 1.2mmol) to the mixed solution, keep it for half an hour, then add parthenolide (248mg, 1.0mmol), raise it to room temperature, keep it for 8 h...
Embodiment 2
[0048] Example 2: Pharmacological effects of parthenolide dithiocarbamate derivatives or their salts
[0049] Match various cancer cells into 2×10 5 / mL cell suspension, add it to the 96-well round-bottom cell culture plate, add parthenolide dithiocarbamate derivatives or their salts, 3 wells for each test concentration, set at 37℃, 5% CO 2 After culturing for 72 hours under saturated humidity conditions, the absorbance (A) value was measured by the MTT method at 570nm wavelength of the enzyme-linked detector, and the inhibitory effect of the compound of the present invention on the tested cancer cells was calculated and repeated three times.
[0050] Table 1 Inhibitory activity of parthenolide dithiocarbamate derivatives on various cancer cells (IC 50 , ΜM)
[0051] Compound
KG1a
HL60
Doxorubicin (positive control)
0.75±0.05
0.022±0.005
6.1±1.8
3.8±1.2
1
8.6±1.1
13.4±2.0
2
4.8±3.2
7.3±1.1
3
16.0±1.1
6.5±2.5
4
50.0±14.0
20.7±4.6
5
13.5±1.0
10.6±2.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com