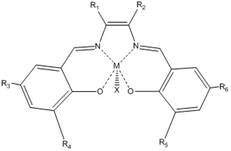
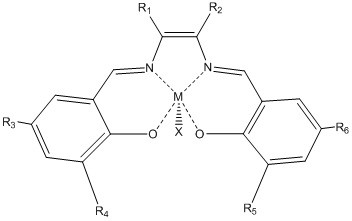
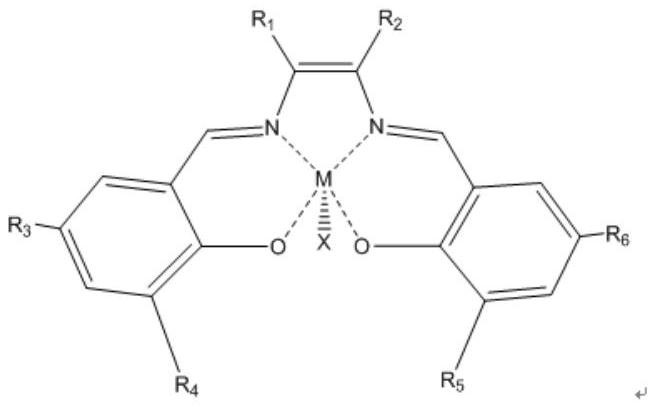
Catalyst for hydration of epoxy compound to diol and its application
A technology of epoxy compounds and catalysts, which is applied in the direction of organic compound/hydride/coordination complex catalysts, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as high cost, poor catalyst stability, and cumbersome preparation steps. Achieve the effect of easy separation, easy preparation and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of the catalyst in the present invention.
[0038]Mix 1 g of sodium dodecylbenzene sulfonate with 0.27 g of tert-butyl methacrylate, 0.28 g of n-butyl methacrylate, 0.33 g of ethylene glycol dimethacrylate, 59 mg of glucose pentaacetate and 20 mg of peroxide Lauroyl was dissolved in 1ml of chloroform to obtain a mixed solution; 1g of hexadecyltrimethyl-p-toluenesulfonium was mixed with 0.27g of tert-butyl methacrylate, 0.28g of n-butyl methacrylate, 0.33g of dimethyl methacrylate Ethylene glycol acrylate, 59 mg of glucose pentaacetate and 25 mg of 4,4'-methylenebis(N,N-dimethylaniline) were dissolved in 1 ml of chloroform to obtain a mixed solution. After volatilizing the solvent in the two mixed solutions and draining the mixture, the mixture was dissolved in 100 ml of 10% Co(Salen)-OTS chloroform solution, and the solution containing sodium dodecylbenzene sulfonate and the solution containing hexadecyl The trimethyl-p-toluenesulfonium ammonium solution wa...
Embodiment 2
[0040] Mix 1 g of sodium dodecylbenzene sulfonate with 0.27 g of tert-butyl methacrylate, 0.28 g of n-butyl methacrylate, 0.33 g of ethylene glycol dimethacrylate, 59 mg of glucose pentaacetate and 20 mg of peroxide Lauroyl was dissolved in 1ml of chloroform to obtain a mixed solution; 1g of hexadecyltrimethyl-p-toluenesulfonium was mixed with 0.27g of tert-butyl methacrylate, 0.28g of n-butyl methacrylate, 0.33g of dimethyl methacrylate Ethylene glycol acrylate, 59 mg of glucose pentaacetate and 25 mg of 4,4'-methylenebis(N,N-dimethylaniline) were dissolved in 1 ml of chloroform to obtain a mixed solution. After volatilizing the solvent in the two mixed solutions and draining to obtain the solid phase, they were dissolved in 100 ml of 20% Co(Salen)-OTS chloroform solution, respectively, and the solution containing sodium dodecylbenzenesulfonate and the solution containing hexadecylbenzene sulfonate were mixed. The alkyl trimethyl-p-toluenesulfonic acid ammonium solution was m...
Embodiment 3
[0042] Mix 1 g of sodium dodecylbenzene sulfonate with 0.27 g of tert-butyl methacrylate, 0.28 g of n-butyl methacrylate, 0.33 g of ethylene glycol dimethacrylate, 59 mg of glucose pentaacetate and 20 mg of peroxide Lauroyl was dissolved in 1ml of chloroform to obtain a mixed solution; 1g of hexadecyltrimethyl-p-toluenesulfonium was mixed with 0.27g of tert-butyl methacrylate, 0.28g of n-butyl methacrylate, 0.33g of dimethyl methacrylate Ethylene glycol acrylate, 59 mg of glucose pentaacetate and 25 mg of 4,4'-methylenebis(N,N-dimethylaniline) were dissolved in 1 ml of chloroform to obtain a mixed solution. After volatilizing the solvent in the two mixed solutions and draining to obtain the solid phase, they were dissolved in 100 ml of 30% Co(Salen)-OTS chloroform solution, respectively, and the solution containing sodium dodecylbenzenesulfonate and the solution containing hexadecylbenzene sulfonate were mixed. The alkyl trimethyl-p-toluenesulfonic acid ammonium solution was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com