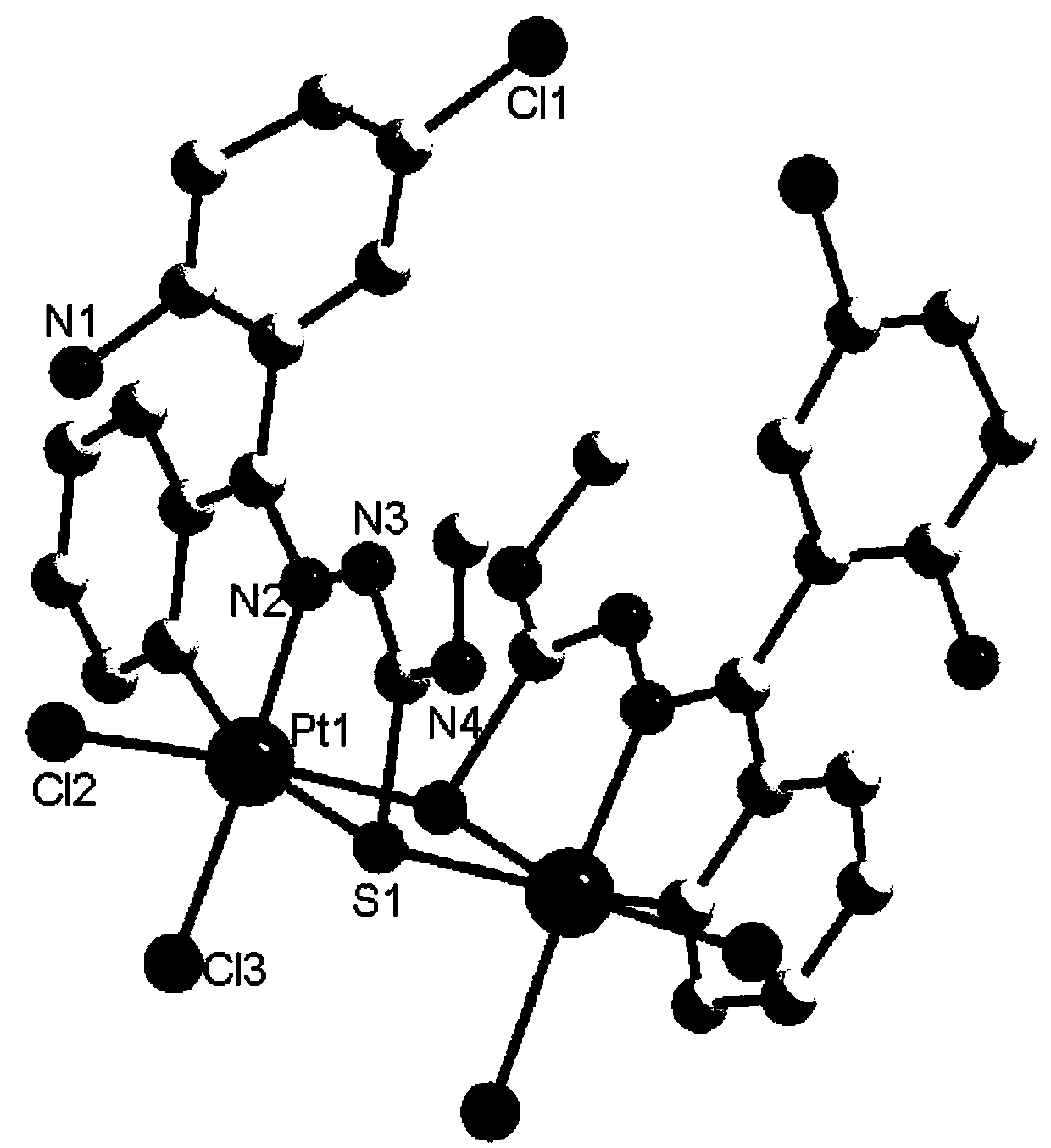
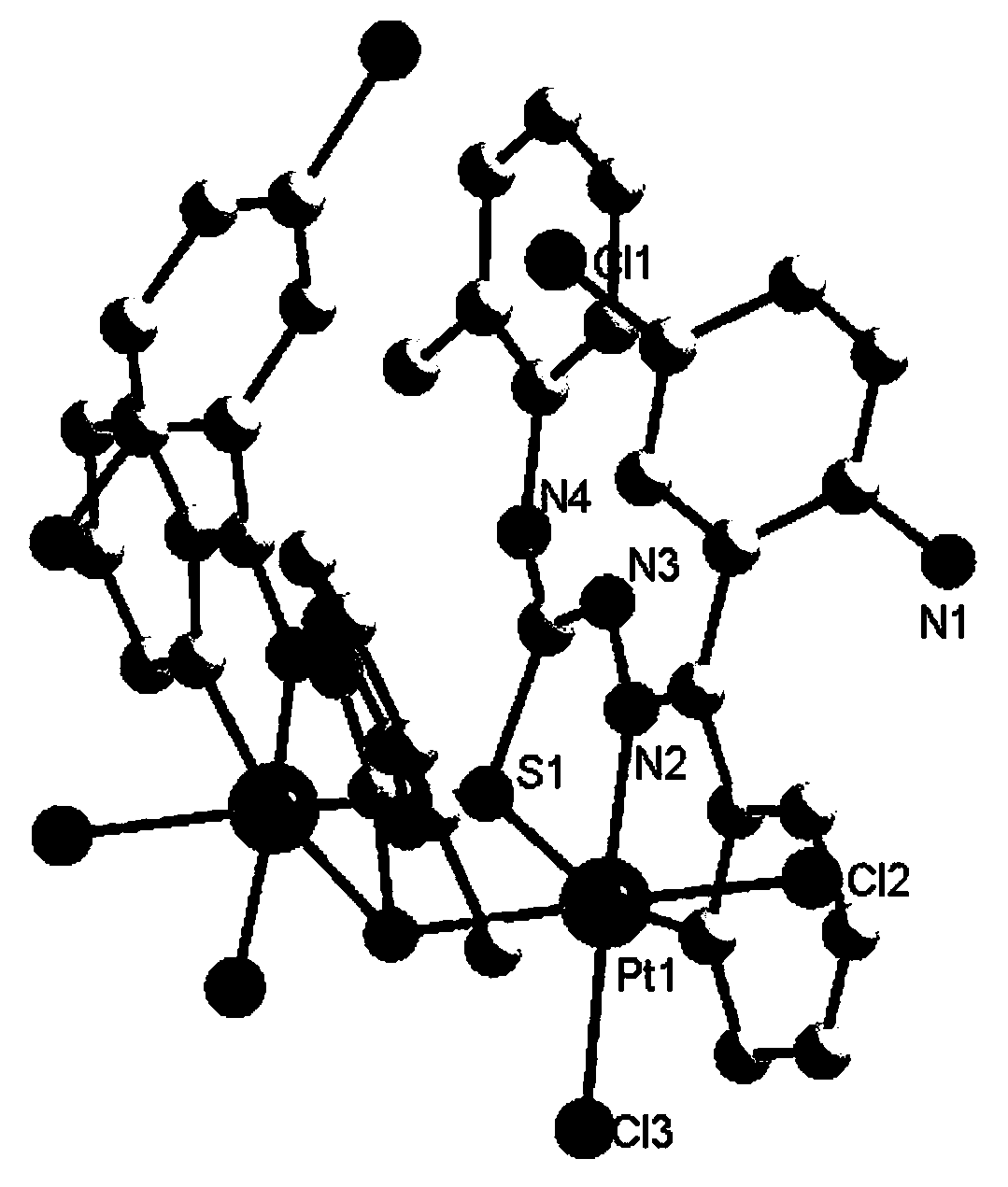
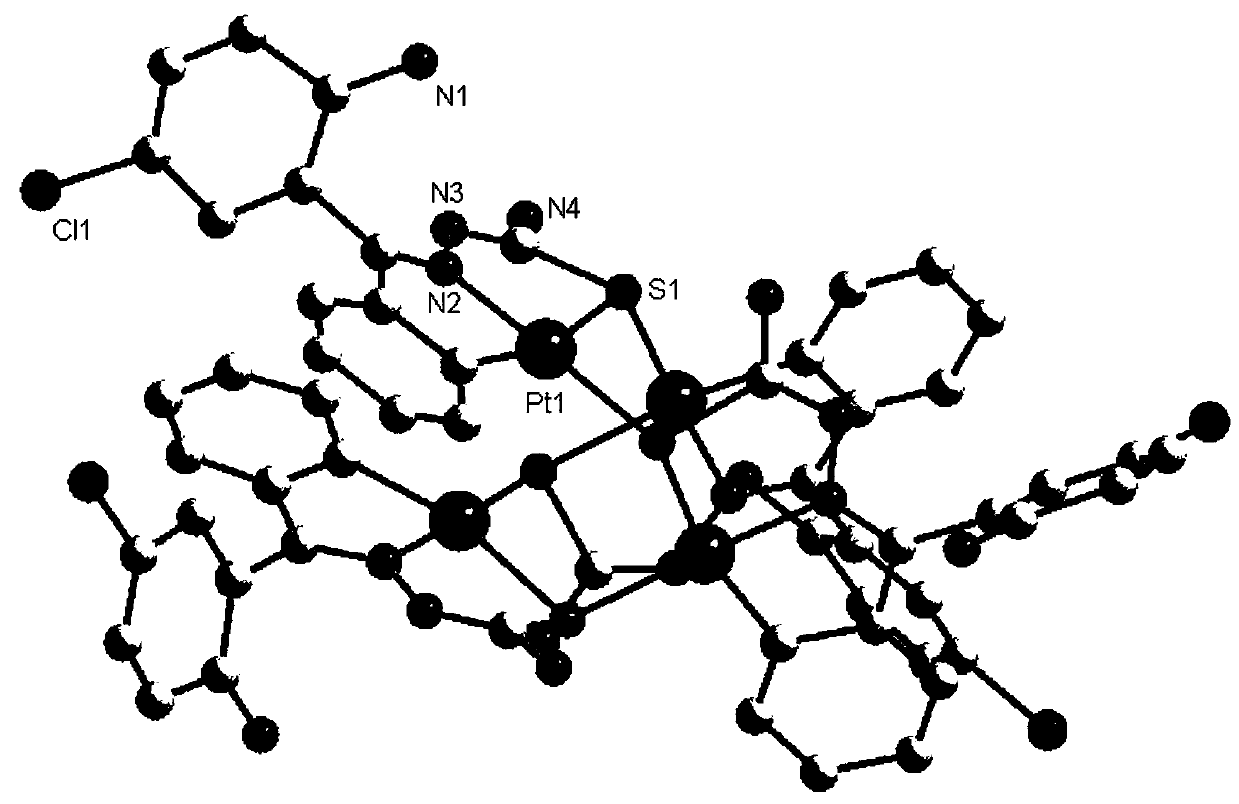
Platinum complex using 2-amino-5-chlorobenzophenone thiosemicarbazone as ligand, and synthesis method and application thereof
A technology of chlorobenzophenone thiosemicarbazone and chlorobenzophenone, which is applied in the field of synthesis of metal complexes, can solve the problems of no anti-tumor activity, achieve novel structure, excellent neuroma inhibitory activity, and overcome drug The effect of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis method of C1 complex is:
[0029] 1) Dissolve 3mmol (693mg) 2-amino-5-chlorobenzophenone in 20mL ethanol, then add 3mmol (315mg) 4-methyl-3-thiosemicarbazide and mix well, add 3-4 drops of concentrated Sulfuric acid was refluxed and filtered, the filtrate was volatilized at room temperature, and light yellow crystals were precipitated, the crystals were filtered, and washed with absolute ethanol for 2-3 times to obtain compound 1;
[0030] Yield: 0.75g, 78.6%, light yellow solid; R f =0.414(Petroleum ether:EtOAc=1:1).M.p.133-135℃. 1 H NMR (400MHz, DMSO-d 6 )δ8.79(q,J=4.5Hz,1H),8.70(s,1H),7.72-7.69(m,2H),7.42-7.37(m,3H),7.29(dd,J=8.8,2.5Hz ,1H),6.92-6.90(m,2H),5.28(s,2H),3.06(d,J=4.6Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ177.72, 145.97, 144.55, 135.86, 130.55, 129.54, 128.25, 127.55, 119.68, 117.44, 116.18, 31.30.ESI + m / z: calcd for C 15 h 16 ClN 4 S, 317 [M-H] + .
[0031] 2) Take 0.05mmol (15.9mg) of compound 1 and 23mg of Pt(DMSO) obtained in s...
Embodiment 2
[0034] The synthesis method of C2 complex is:
[0035] 1) Dissolve 3mmol (693mg) 2-amino-5-chlorobenzophenone in 20mL ethanol, then add 3mmol (543mg) 4-(2-methylphenyl)-3-thiosemicarbazide and mix well, drop Add 3-4 drops of concentrated sulfuric acid, reflux, and filter, the filtrate is volatilized at room temperature, and pale yellow crystals are precipitated, the crystals are filtered, and washed 2-3 times with absolute ethanol to obtain compound 2;
[0036] Yield: 0.71g, 60.0%, light yellow solid; R f =0.421 (Petroleum ether:EtOAc=2:
[0037] 1).M.p.140-142℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.23(s,1H),9.04(s,1H),7.82-7.79(m,2H),7.43-7.37(m,3H),7.33-7.29(m,3H),7.25-7.22(m, 2H),6.99-6.93(m,2H),5.39(s,2H),2.27(s,3H). 13 C NMR (100MHz, DMSO-d 6)δ176.99,146.83,144.63,137.91,135.82,135.24,130.63,130.08,129.68,128.40,128.29,128.23,127.88,126.83,125.93,119.76,117.50,1176.7 + m / z: calcd for C 21 h 18 ClN 4 S, 393 [M-H] + .
[0038] 2) Take 0.05mmol (15.2mg) of compound 2 a...
Embodiment 3
[0041] The synthetic method of C3 complex is:
[0042] 1) Dissolve 3mmol (693mg) 2-amino-5-chlorobenzophenone in 20mL ethanol, then add 3mmol (273mg) thiosemicarbazide and mix well, add 3-4 drops of concentrated sulfuric acid, reflux, filter, and the filtrate Volatilize at room temperature, and a light yellow crystal precipitates out. The crystal is filtered and washed with absolute ethanol 2-3 times to obtain compound 3;
[0043] Yield: 0.76g, 83.3%, light yellow solid; R f =0.321(Petroleum ether:EtOAc=1:1).M.p.158-160℃. 1 H NMR (400MHz, DMSO-d 6 )δ8.61(s,2H),8.28(s,1H),7.72-7.69(m,2H),7.41-7.35(m,3H),7.28(dd,J=8.8,2.6Hz,1H),6.92 -6.90(m,2H),5.29(s,2H). 13 CNMR (100MHz, DMSO-d 6 )δ178.09, 146.40, 144.57, 135.80, 130.57, 129.60, 128.27, 127.63, 119.68, 117.45, 116.12.ESI + m / z: calcd for C 14 h 14 ClN 4 S, 303 [M-H] + .
[0044] 2) Take 0.05mmol (17.9mg) of compound 3 and 23mg of Pt(DMSO) obtained in step 1) 2 Cl 2 Place in a glass tube with one end sealed, add 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com