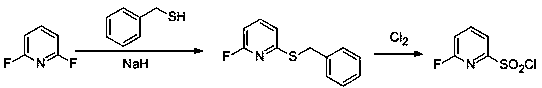
Method for preparing 6-fluoro-2-pyridinesulfonyl chloride
A technology of pyridinesulfonyl chloride and difluoropyridine difluoropyridine is applied in the field of preparing 6-fluoro-2-pyridinesulfonyl chloride, which can solve the problems of potential safety hazards, use safety risks, high toxicity and the like, and achieves improved safety and reliability. The effect of operability, reduction of raw material cost and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
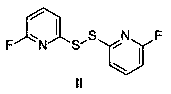
[0012] Embodiment 1 (preparation of formula II)
[0013] Add 100g of water, 20g of sodium sulfide, and 8.2g of sulfur powder into the reaction flask, and heat the reaction until the sulfur powder dissolves to obtain an orange sodium disulfide solution. After cooling to room temperature, add 19.6 g of 2,6-difluoropyridine and ethanol to the reaction flask, heat the reaction until the raw materials disappear, add tertiary methyl ether for extraction after cooling, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a yellow oil. The yield was 70%, and the purity of the product detected by HPLC was 92.2%.
Embodiment 2
[0014] Embodiment 2 (preparation of formula II)
[0015] Add 60g of water, 11.2g of sodium hydrosulfide, 50g of methanol, 4g of 2,6-difluoropyridine into the reaction flask, heat the reaction until the raw materials disappear, add tertiary methyl ether after cooling for extraction, dry over anhydrous sodium sulfate, filter, concentrate, A yellow oil was obtained with a yield of 52%, and the purity of the product detected by HPLC was 85.7%.
Embodiment 3
[0016] Embodiment 3 (preparation of formula II)
[0017] Add 150g of water, 23g of sodium sulfide, 60g of N,N-dimethylformamide, 5g of 2,6-difluoropyridine into the reaction flask, heat the reaction until the raw materials disappear, add tertiary methyl ether for extraction after cooling, anhydrous sodium sulfate Drying, filtration, and concentration gave a yellow oil with a yield of 33%. The purity of the product was 94.2% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com