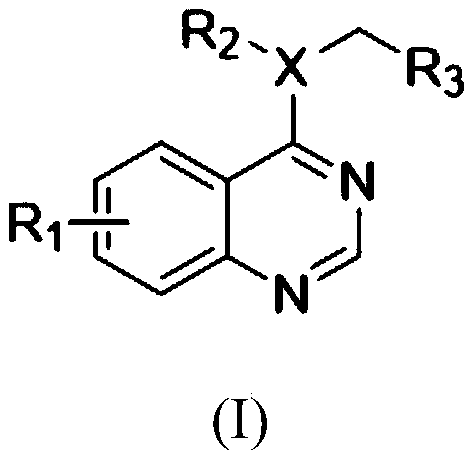
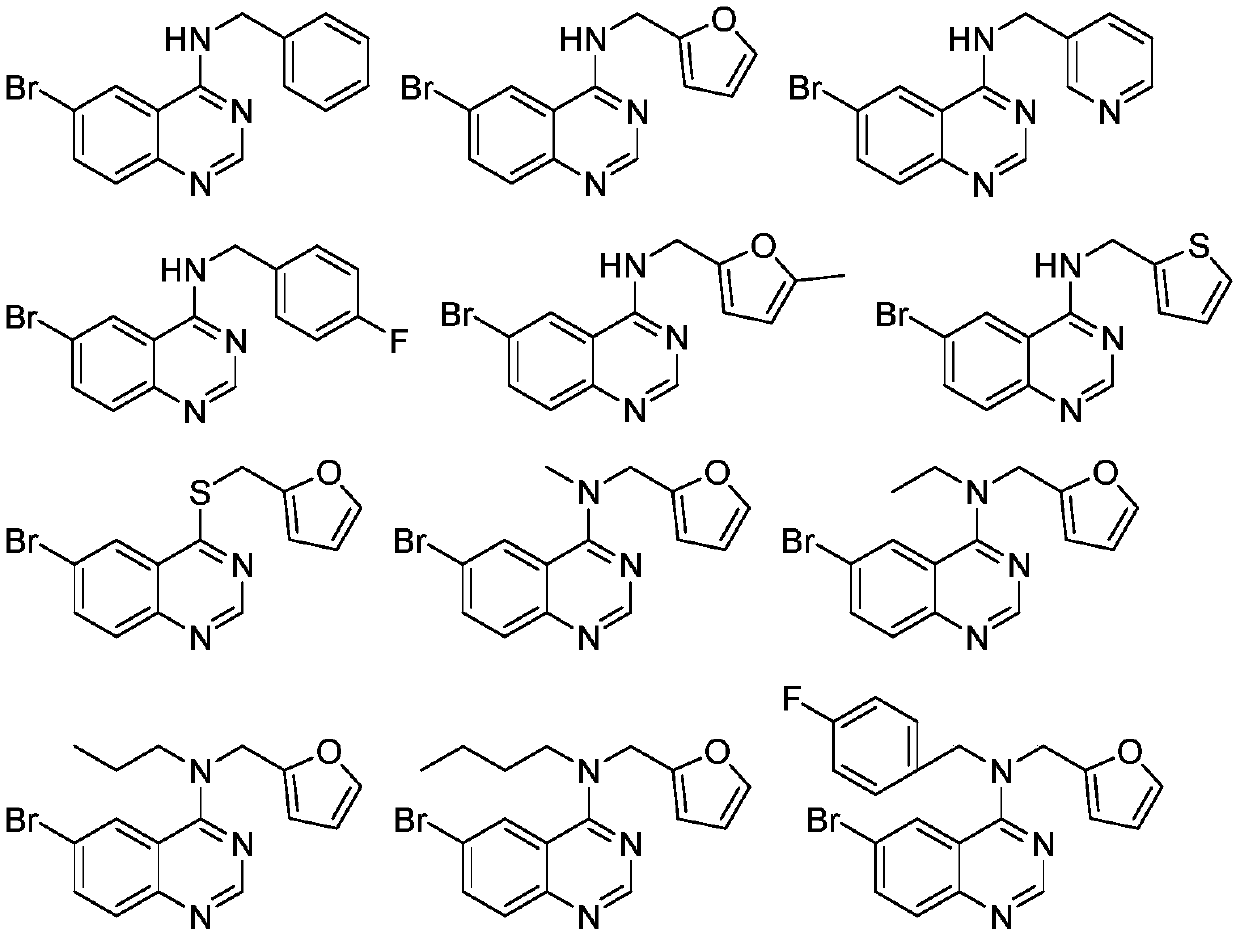
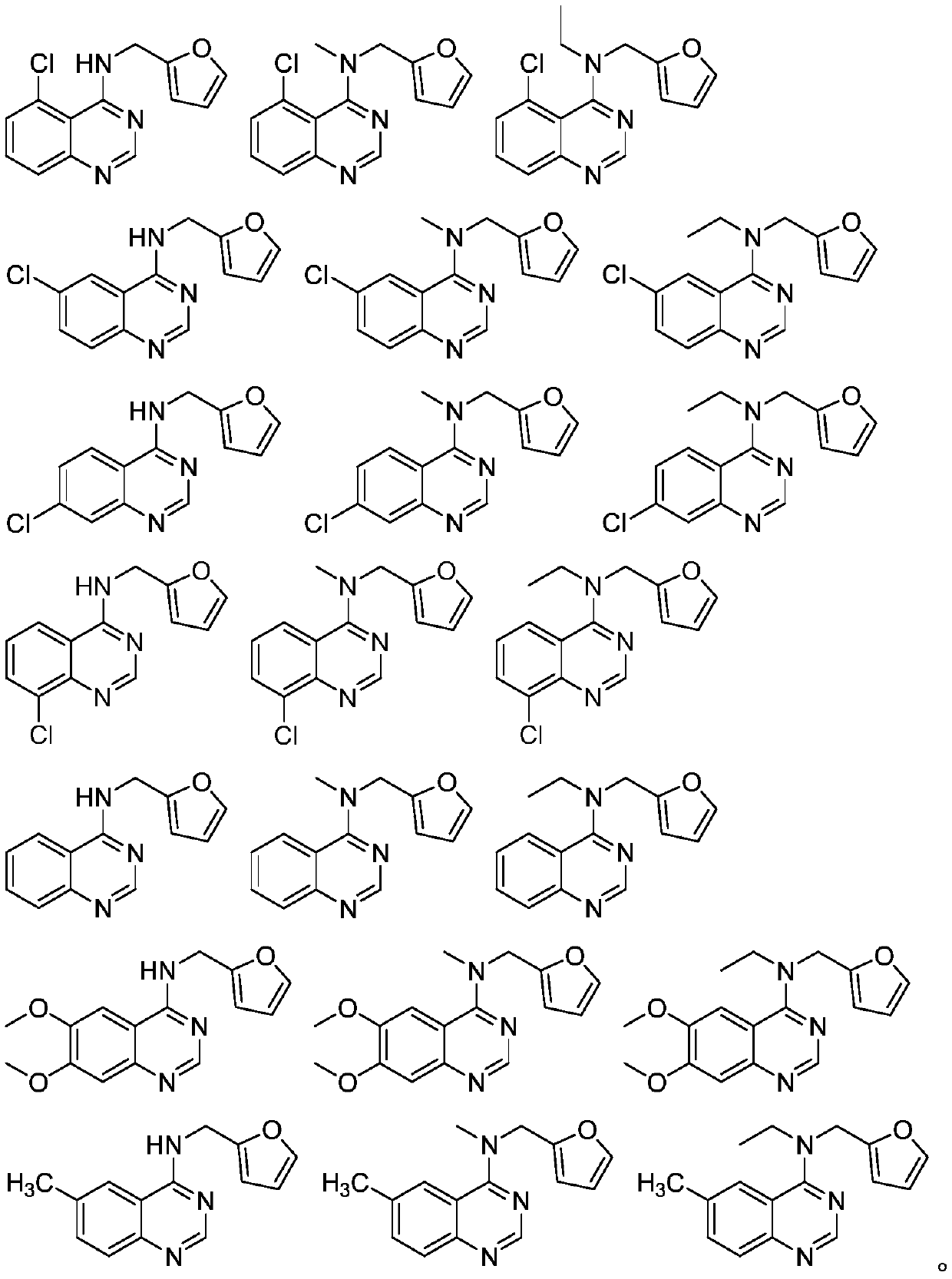
4-Amino quinazoline compound as well as preparation method and application thereof
A technology for aminoquinazolines and compounds, which is applied in the field of quinazoline compounds substituted by aromatic ring methylene groups and their preparation, and can solve the problems of agricultural production loss, insufficient activity of commercialized antibacterial agents, and serious harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the preparation of 5-chloroquinazolin-4 (3H)-one
[0061] Put 6-chloro-2-aminobenzoic acid (87.0mmol) in a 100mL round-bottomed flask, add 30mL formamide to it and stir, heat to 145°C for 5h, then stop the reaction, cool to room temperature, filter with suction, wash with water to obtain off-white Solid, the yield after drying is 76.0%.
Embodiment 2
[0062] Embodiment 2: the preparation of 4,5-dichloroquinazole
[0063] Put 5-chloroquinazolin-4(3H)-one (11.0mmol) in a 100mL round bottom flask, add 20mL of toluene to it and stir to dissolve, then add 5 equivalents of POCl to the system 3 and 2 equivalents of triethylamine, heated to reflux at 120°C, reacted for 5 hours, stopped the reaction, dissolved the solid with 100mL DCM, adjusted the pH to 6-7 with ammonia water, washed with water (30mL*3), and purified by column chromatography ( PE:EA=10:1), a white solid was obtained with a yield of 54.4%.
Embodiment 3
[0064] Embodiment 3: Preparation of 5-chloro-N-(furan-2-methylene)quinazolin-4-amine
[0065] Add 5-chloroquinazoline (5mmol) into a 50mL round bottom flask, add 20mL of dry isopropanol and stir to dissolve, then add 1.5 equivalents of triethylamine and 1.2 equivalents of 2-furylmethylamine, react at 80°C for 1 hour, then stop the reaction , precipitation, and column chromatography (eluent PE:EA=15:1) to obtain a white solid with a yield of 76.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com