A kind of method and application thereof for preparing phthaloyl amlodipine
A technology of phthaloyl ammonia chloride and dipine, applied in the direction of organic chemistry and the like, can solve the problems of long route, low final yield, complicated process and the like, and achieve the effects of short process, small amount of waste water and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Example 1 Preparation of phthal amine chlorine chloride
[0039]1) 50 g of dihydropyridine, silica 3A molecular sieve 4g, N-bromide-butilimide (NBS) 70 g, stirred, 80 ° C is heated, and the reaction is 60 ° C, and the reaction is completed, stirred after the reaction is completed. To room temperature, it was stirred in an ice water bath for 1 h, filtered, and dried under vacuum at 50 ° C for 12 h, resulting in 74 g of a pale yellow solid powder (structural formula II), the crude rate of 90%, purity 98.375%.
[0040]2) 1000ml round bottom flask is added to 74 g, N-hydroxyethyl phthalamine 37.4 g, N-hydroxyethyl phthalamine 37.4 g, potassium carbonate 13g, N, N-dimethylformamide 1.3 g, methyl ethyl 300g, stirring, heat reflux at 80 ° C, reaction 8 h; After the reaction was completed, 200 g of water was added, stirred for 1 h, stirred, filter cake 50 ° C for 12 h, resulting 87.7 g of white solid powder (Structure III) ), The crude product rate was 95%, purity 97.932%.
[0041]3) 1000ml ro...
Embodiment 2
[0043]Example 2 Preparation of phthalophyllocytex form
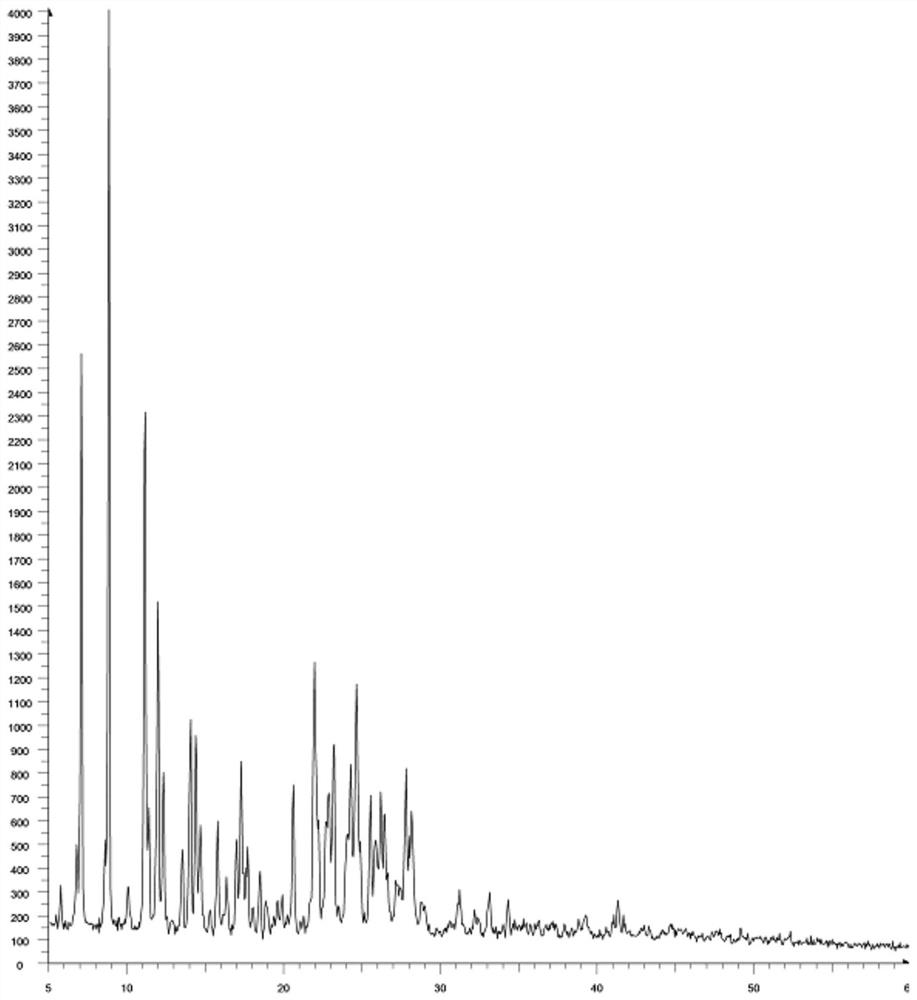
[0044]The pale yellow solid powder 50g, a acetate, 400 g of ethyl acetate, stirred, stirred, stirred to 60 ° C, stirred, stirred, stirred, stirred to be heated to room temperature, stirred, stirred, stirred, stirred at room temperature, stirred with 50 g of ethanol, stir 2H, filtration, filter cake 50 ° C for 12 h, resulting in 49.6 g of pale yellow solid powder (phthalophenylel aminogenic crystalline type A), a crude rate of 99.2%, purity 99.758%. The phrochlorphrochlorine chloride formation A, the first melter was 138.1 ° C, and 138.7 ° C.
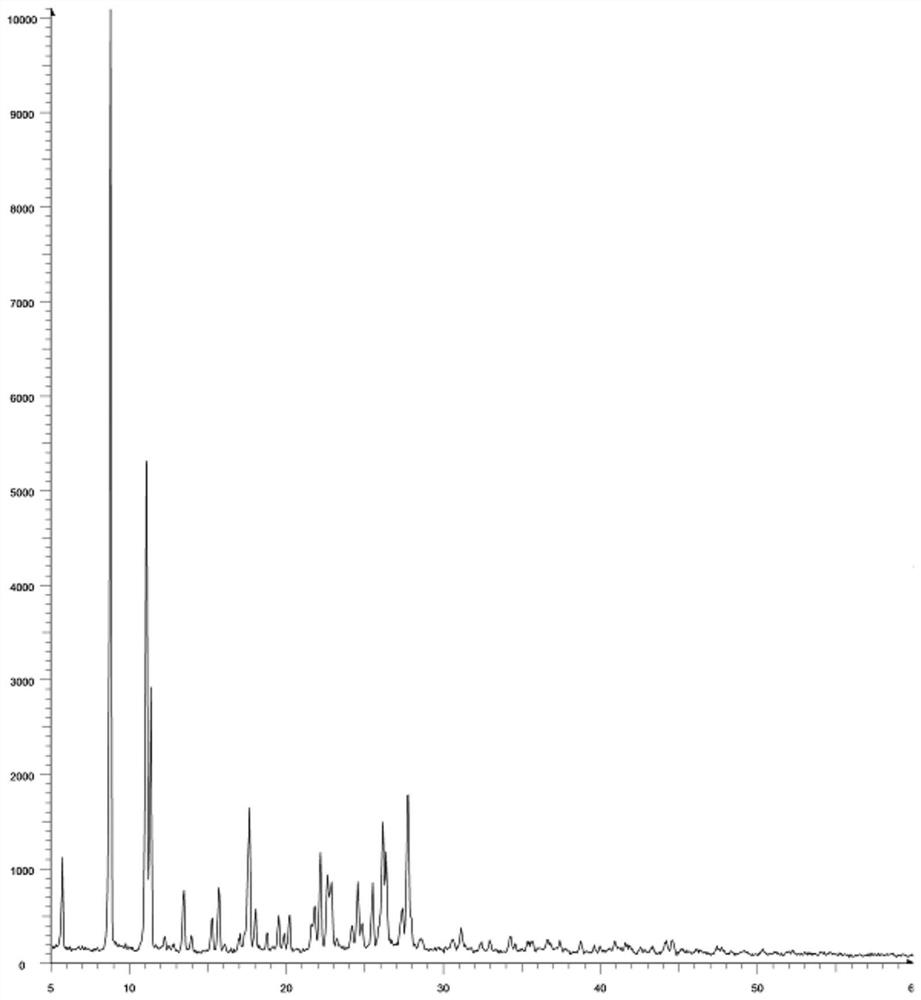
[0045]The X-ray pattern peak of phthalophyllocytex form Afigure 1 As shown: 5.7, 1107; 8.7, 10103; 11.3, 2912; 13.4, 759; 15.2, 463; 15.6, 786; 17.6, 1633; 18.0, 561; 19.5, 490; 20.2, 498; 21.8, 589 22.1,1159; 22.6,923; 24.5,849; 25.5,839; 26.1,1482; 26.3, 1167; 27.4,569; 27.7,1772.
Embodiment 3
[0046]Example 3 Preparation of phthalophylline Crystalline Type B
[0047]Add 49.6 g of the above pale yellow solid powder, add N, N-dimethylformamide, stirred, stirred to 60 ° C, stirred to 60 ° C, stirred to 60 ° C, stirred to heating, 0.20 g of water, stirred with 20 g of water, stir for 2 h, The solid was completely precipitated, filtered, and the filter cake was dried in vacuo for 12 h, resulting in 49.4 g of a pale yellow solid powder (phthal amyline chlorine), the crude rate of 99.6%, purity 99.862%. The o-phenylenedicanoyl amchlopine chloride was determined, the first melting was 147.2 ° C, and the final melted was 148.8 ° C.
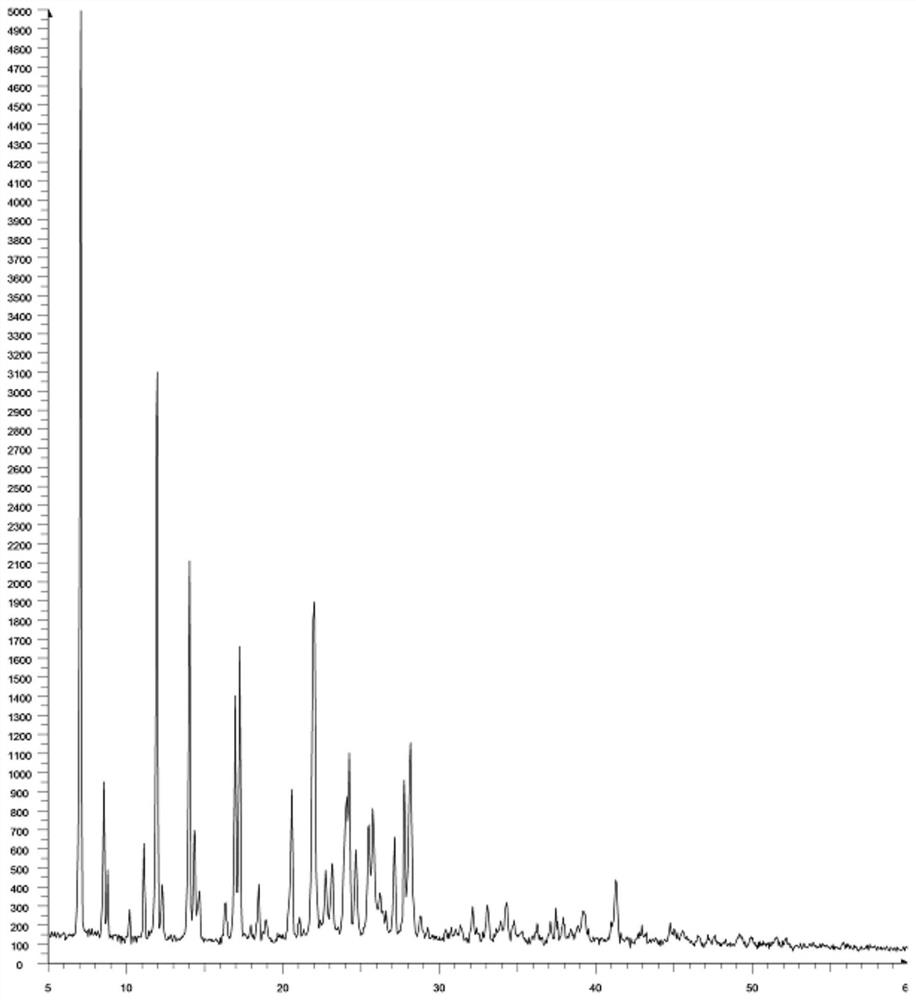
[0048]X-ray map of amnoblobacine amine is likefigure 2 As shown: 7.0,5001; 8.5,945; 8.7,483; 10.1,274; 11.1,622; 11.9,3099; 12.2,405; 14.0,2107; 14.3,691; 14.6,371; 16.3,310; 16.9,1398 17.2,1656; 18.4,406; 20.5,905; 22.0,1891; 22.7,482; 23.1,515; 24.1,868; 24.2,1097; 24.6,588; 25.5,719; 25.7,804; 27.1,655; 27.7 , 954; 28.1, 1151.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com