Rh-FSH preparation and preparing method thereof
A technology of rh-fsh and preparation, applied in the field of human reproduction, can solve the problems of poor freeze-drying effect, poor reconstitution effect of freeze-dried preparations, etc., and achieves the effect of easy freeze-drying process and good freeze-drying process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A rh-FSH preparation consisting of:
[0051]
[0052] The above components were further processed as follows.
[0053] Step 1: Dosing
[0054] (1) Take an appropriate amount of water for injection, slowly add other ingredients except rh-FSH into the water for injection, and stir until completely dissolved.
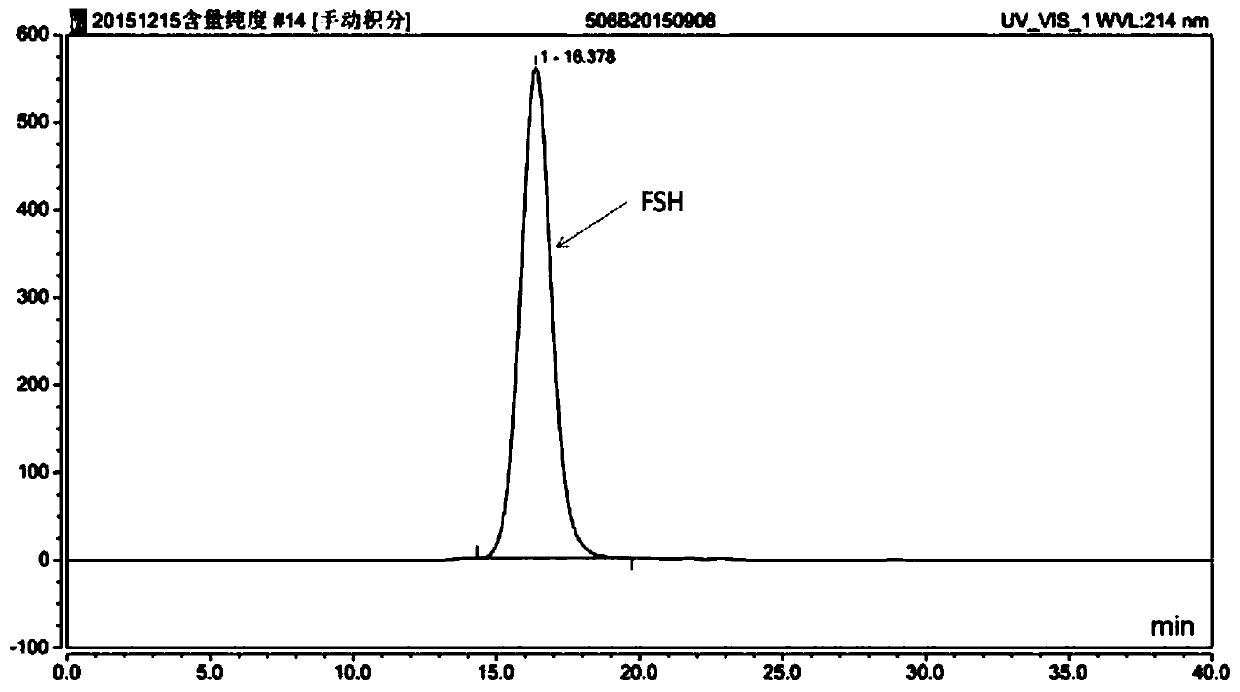
[0055](2) Finally, add the rh-FSH stock solution and mix well, and adjust the pH value to pH 7.0 ± 0.5 with phosphoric acid / sodium hydroxide. At this time, the rh-FSH content is about 5.5 μg / ml.
[0056] (3) Pass the prepared solution through a 0.2 μml filter membrane to sterilize and filter, put 1ml ± 0.02ml into 2ml vials in a sterile environment, and freeze-dry according to the following steps.
[0057] Step 2: Freeze drying
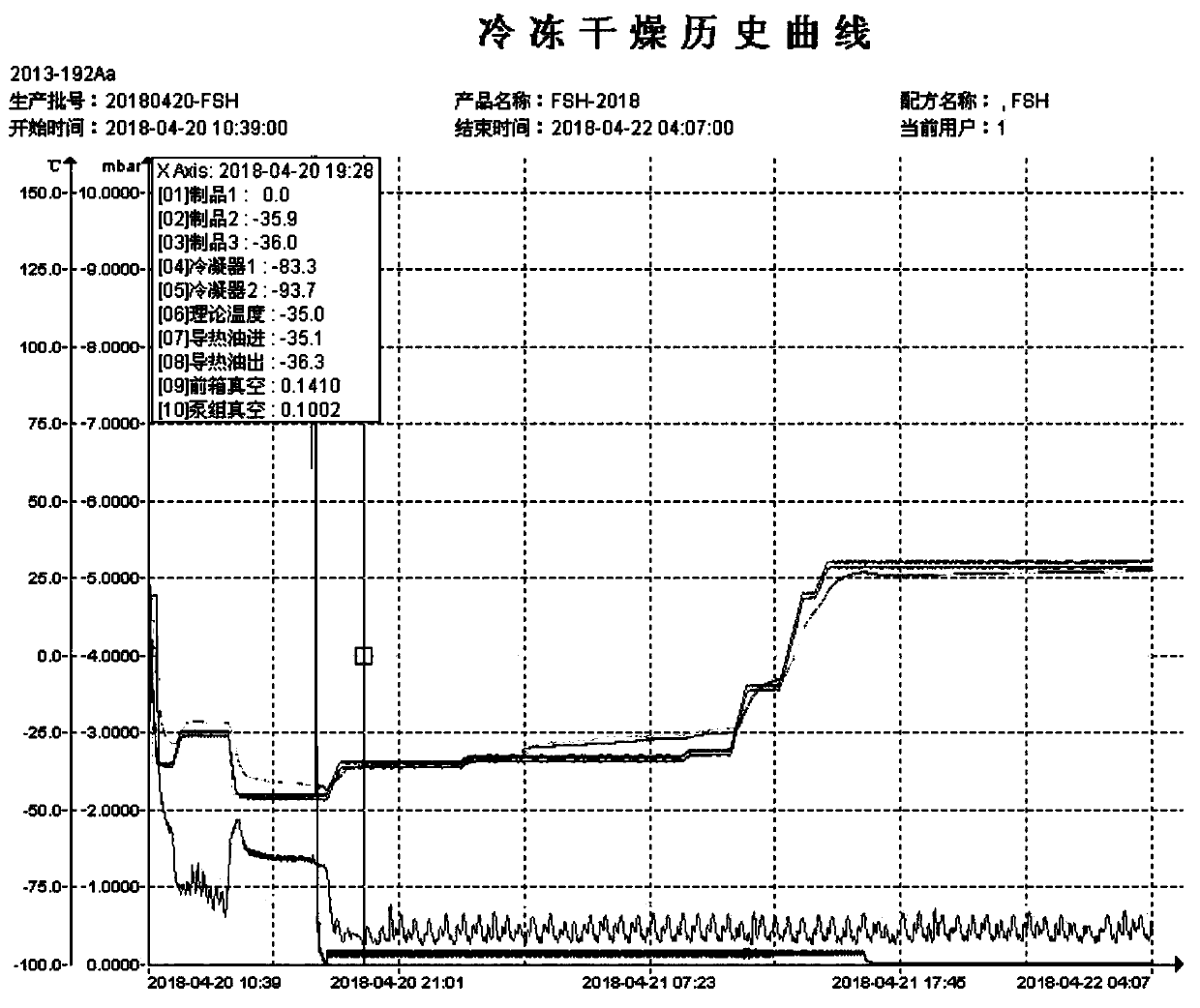
[0058] (1) Pre-freezing step: gradually lower the temperature to -35 degrees, pre-freeze for 40 minutes, then raise the temperature to -25 degrees, keep for 100 minutes, then lower the temperature to -45 degrees, and keep for 180 minu...
Embodiment 2
[0063] A rh-FSH preparation consisting of:
[0064]
[0065] The above components were further processed as follows.
[0066] Step 1: Dosing
[0067] (1) Take an appropriate amount of water for injection, slowly add other ingredients except rh-FSH into the water for injection, and stir until completely dissolved.
[0068] (2) Finally, add the rh-FSH stock solution and mix well, and adjust the pH value to pH 7.0 ± 0.5 with phosphoric acid / sodium hydroxide. At this time, the rh-FSH content is about 5.5 μg / ml.
[0069] (3) Pass the prepared solution through a 0.2 μml filter membrane to sterilize and filter, put 1ml ± 0.02ml into 2ml vials in a sterile environment, and freeze-dry according to the following steps.
[0070] Step 2: Freeze drying
[0071] (1) Pre-freezing step: gradually lower the temperature to -35 degrees, pre-freeze for 40 minutes, then raise the temperature to -25 degrees, keep for 100 minutes, then lower the temperature to -45 degrees, and keep for 180 min...
Embodiment 3
[0076] A rh-FSH preparation consisting of:
[0077]
[0078] The above components were further processed as follows.
[0079] Step 1: Dosing
[0080] (1) Take an appropriate amount of water for injection, slowly add other ingredients except rh-FSH into the water for injection, and stir until completely dissolved.
[0081] (2) Finally, add the rh-FSH stock solution and mix well, and adjust the pH value to pH 7.0 ± 0.5 with phosphoric acid / sodium hydroxide. At this time, the rh-FSH content is about 5.5 μg / ml.
[0082] (3) Pass the prepared solution through a 0.2 μml filter membrane to sterilize and filter, put 1ml ± 0.02ml into 2ml vials in a sterile environment, and freeze-dry according to the following steps.
[0083] Step 2: Freeze drying
[0084] (1) Pre-freezing step: gradually lower the temperature to -35 degrees, pre-freeze for 40 minutes, then raise the temperature to -25 degrees, keep for 100 minutes, then lower the temperature to -45 degrees, and keep for 180 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com