Lithium aluminum hydrotalcite-derived nickel-based catalysts for autothermal reforming of acetic acid to hydrogen
A nickel-based catalyst, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen, etc., can solve the problem of catalyst deactivation, easy to be oxidized, not resistant to sintering and other problems, to achieve the effect of promoting gasification reaction, promoting adsorption activation, and improving dispersion degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Weigh 2.322g of Ni (NO 3 ) 2 ·6H 2 O, 22.609g of Al(NO 3 ) 2 9H 2 O and 1.527 g of LiNO 3 , add 90.3ml of deionized water to prepare solution #1; weigh 28.930g of NaOH and 4.791g of anhydrous Na 2 CO 3 , adding 200.8ml of deionized water to prepare solution #2; at 48°C and pH of the solution at 8.0±0.5, add solution #1 and solution #2 dropwise into the beaker and keep stirring for co-precipitation reaction, and Continue stirring and aging for 12 hours; after the aging, the mixture is suction filtered and washed three times, and the obtained precipitate is dried in a 105°C drying oven for 24 hours; the precipitate is calcined at 750°C for 4 hours to obtain CDUT-LNA-101 catalyst. The molar composition of the catalyst is (LiO 0.5 ) 1.47 (NiO) 0.53 (AlO 1.5 ) 4.00 , and the composition in weight percent is as follows: lithium oxide is 8.0%, nickel oxide is 15.0%, and aluminum oxide is 77.0%.
[0030] The reactivity evaluation of autothermal reforming of acetic ...
Embodiment 1
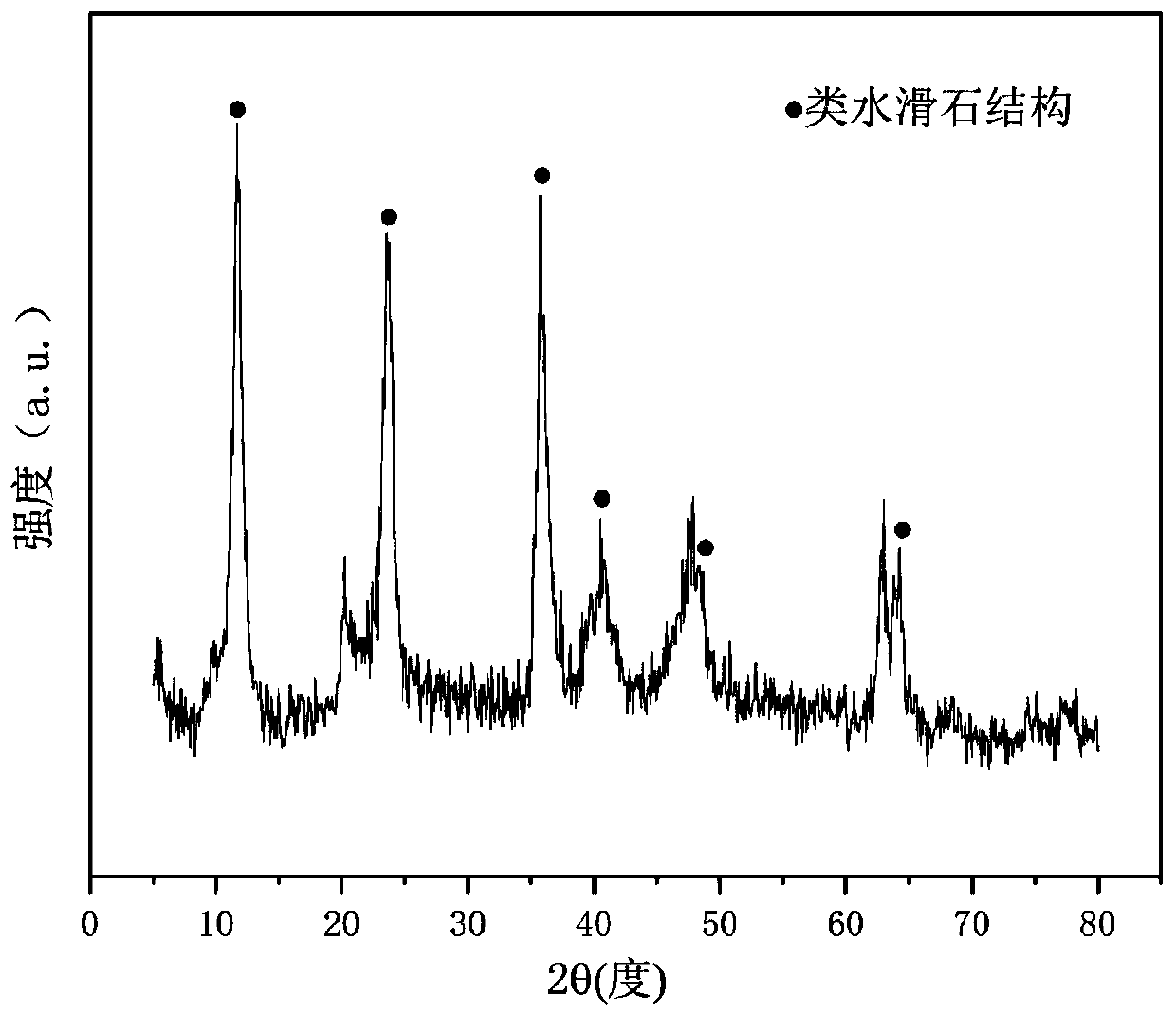
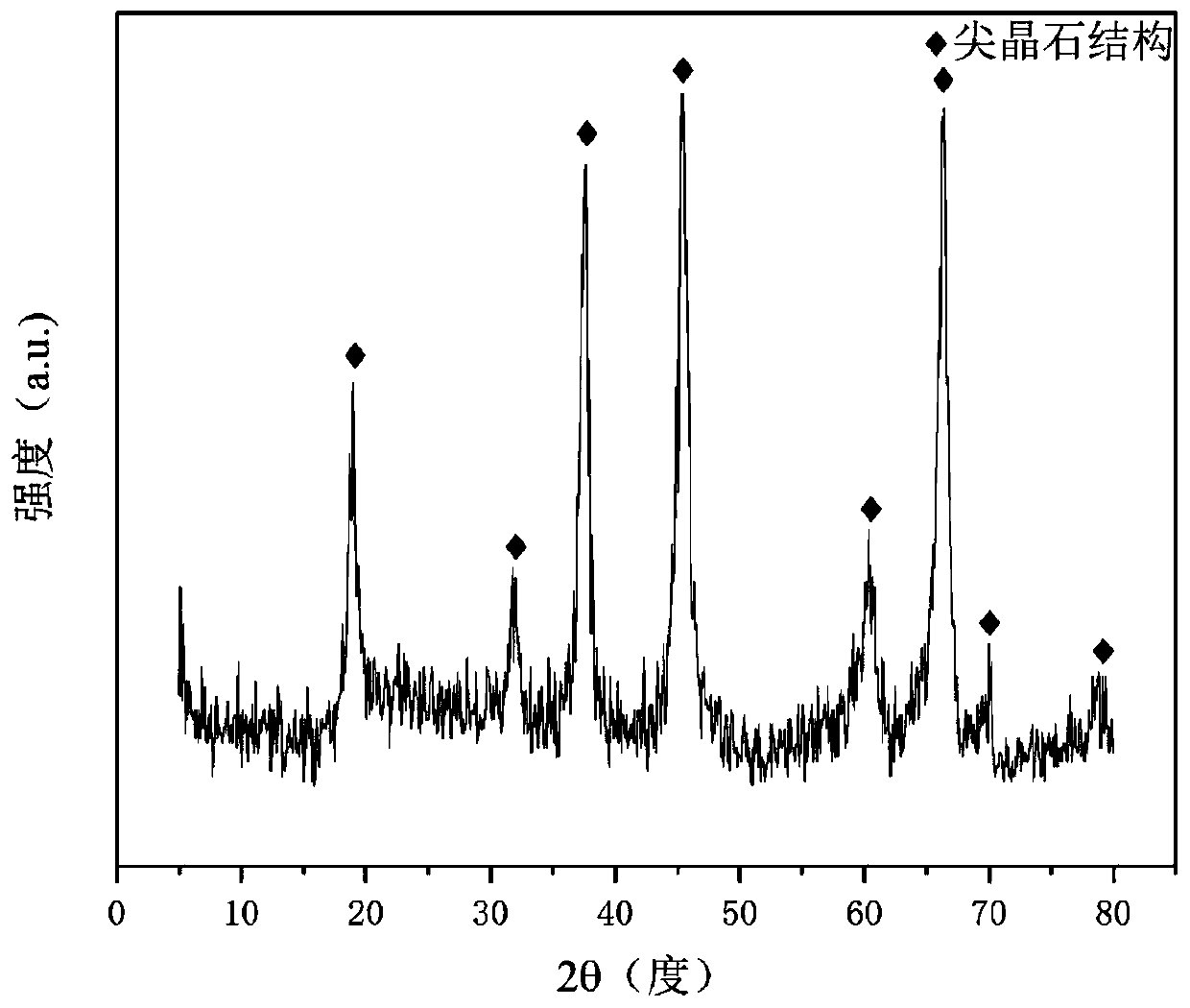
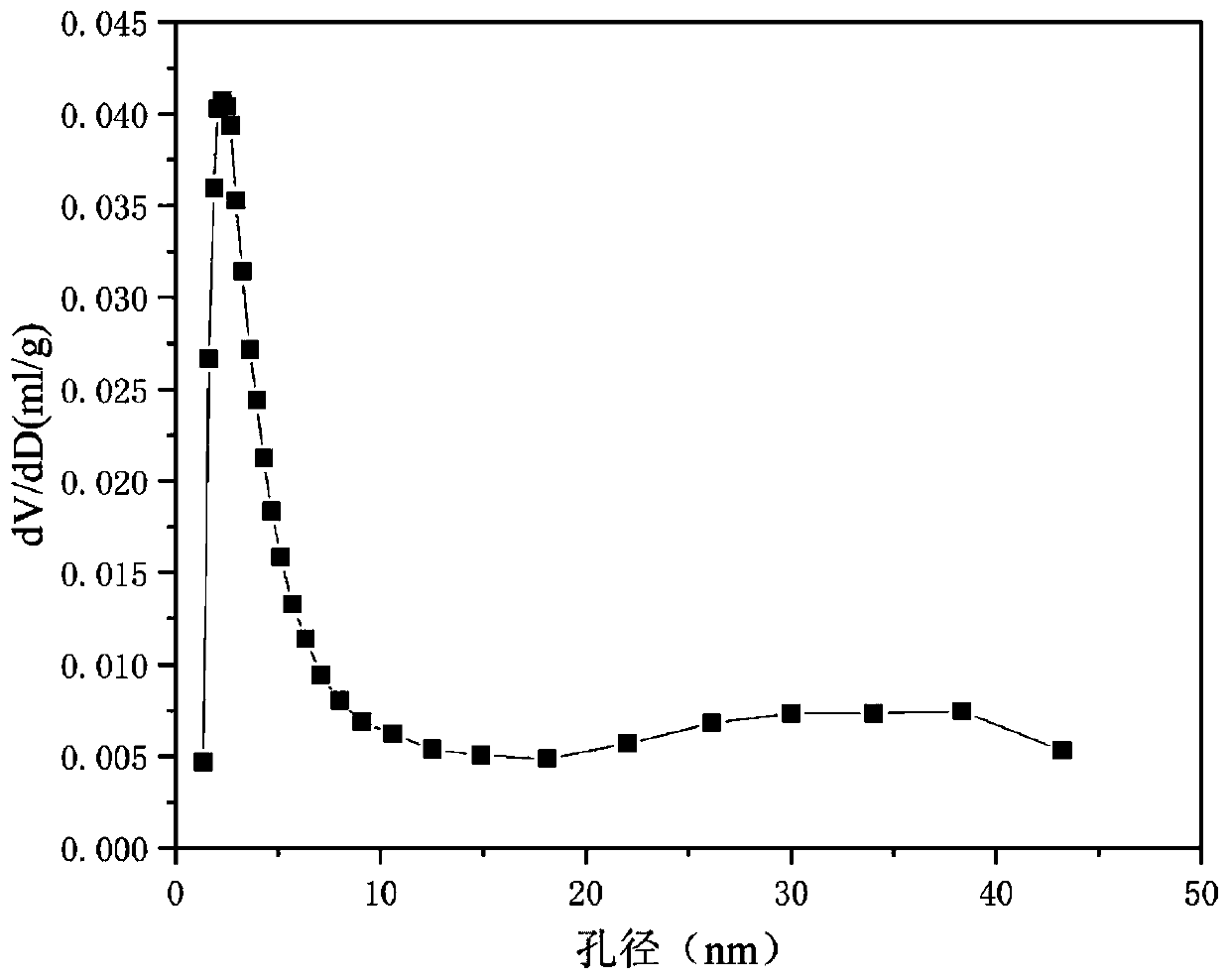
[0033] Weigh 2.369g of Ni (NO 3 ) 2 ·6H 2 O, 16.299g of Al(NO 3 ) 2 9H 2 O and 5.250 g of LiNO 3 , add 130.7ml of deionized water to prepare solution #1; weigh 8.342g of NaOH and 1.381g of anhydrous Na 2 CO 3 , add 221.8ml of deionized water to prepare solution #2; the subsequent steps are the same as in Reference Example 1 to obtain a precursor of hydrotalcite-like structure, and its typical structure is shown in the attached figure 1 shown; Li-Ni-Al-O composite oxide of nickel-aluminum spinel partially substituted by lithium was obtained after calcination, and its typical structure is shown in the attached figure 2 shown. Promptly obtain CDUT-LNA-102 catalyst; This catalyst is a mesoporous material, and its typical pore size distribution is as attached image 3 shown. The molar composition of the catalyst is (LiO 0.5 ) 3.63 (NiO) 0.38 (AlO 1.5 ) 2.00 , and the composition in weight percent is as follows: lithium oxide is 17.0%, nickel oxide is 15.0%, and alum...
Embodiment 2
[0036] Weigh 2.330g of Ni(NO 3 ) 2 ·6H 2 O, 20.038g of Al(NO 3 ) 2 9H 2 O and 3.130 g of LiNO 3 , add 160.3ml of deionized water to prepare solution #1; weigh 34.187g of NaOH and 5.661g of anhydrous Na 2 CO 3 , adding 200.7ml of deionized water to prepare solution #2; the subsequent steps are the same as in Reference Example 1 to obtain a precursor of hydrotalcite-like structure, and its typical structure is shown in the attached figure 1 As shown, Li-Ni-Al-O composite oxide of nickel-aluminum spinel partially substituted by lithium was obtained after calcination, and its typical structure is shown in the attached figure 2 As shown, promptly obtain CDUT-LNA-103 catalyst. The molar composition of the catalyst is (LiO 0.5 ) 3.40 (NiO) 0.60 (AlO 1.5 ) 4.00 , The weight percentage is: lithium oxide is 30.0%, nickel oxide is 15.0%, and aluminum oxide is 55.0%.
[0037] The activity of the CDUT-LNA-103 catalyst was investigated by the autothermal reforming reaction of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com