Tridentate pyridyl imine iron-based catalyst and preparation method and application thereof
A technology of tridentate pyridinium iron series and tridentate pyridinium iron, which is applied in the field of catalytic polymerization of conjugated dienes, can solve problems that have not been reported, and achieve high tolerance, simple preparation, and easy preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
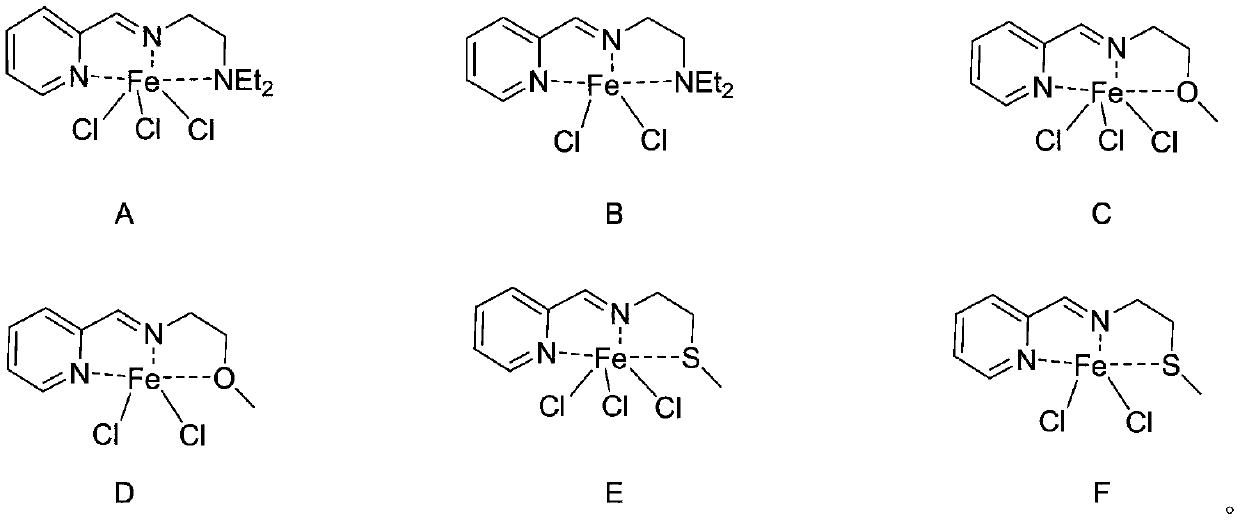
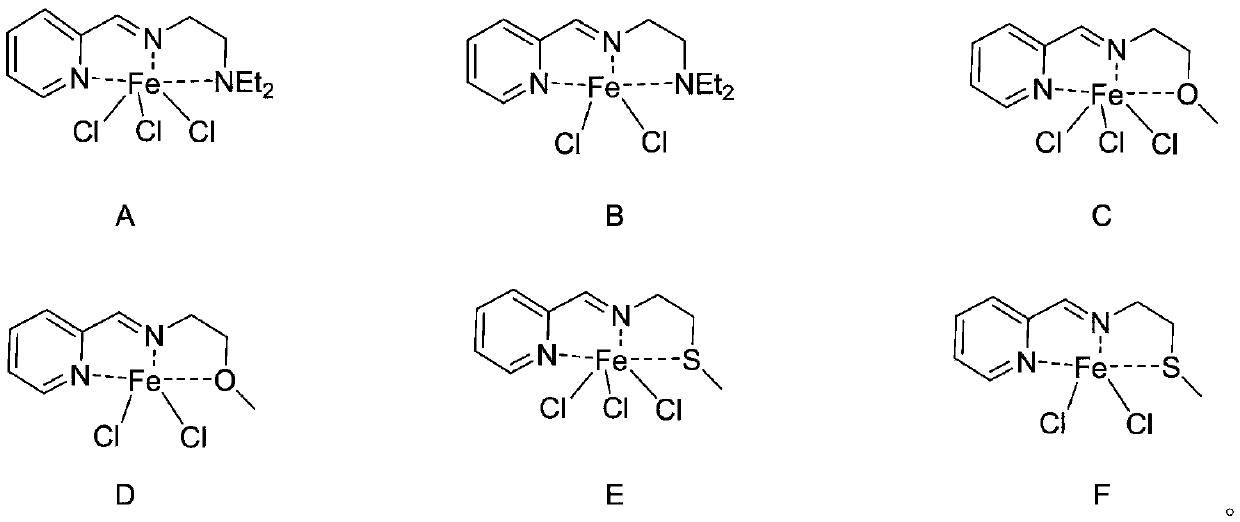
[0031] This example prepares the tridentate pyridinium iron complex shown in formula (A):
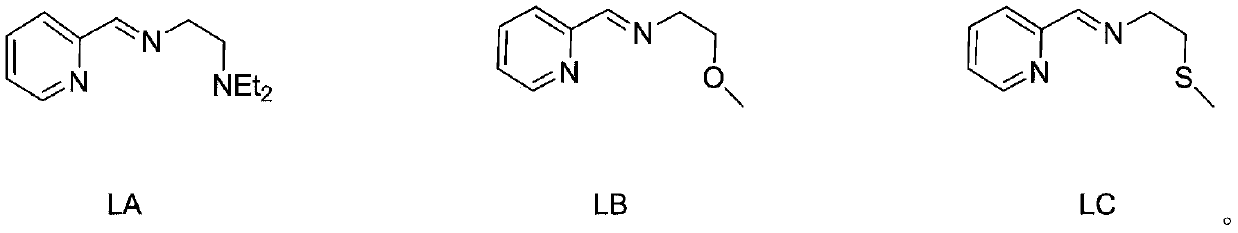
[0032] The 25mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl 3 and tridentate pyridine imine ligand substituted by diethylaminoethyl (1.5 mmol) (structural formula LA), and stirred at room temperature for 24 h. After the reaction, the dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain an off-white solid, structural formula:
[0033]
[0034] Mass Spectrometry: C 12 h 19 Cl 3 FeN 3 :[M-Cl] + : Theoretical value: 331.0300; measured value: 331.0305.
[0035] Elemental Analysis: C 12 h 19 Cl 3 FeN 3 : Theoretical value: C, 39.22%; H, 5.21%; N, 11.43%; Measured value: C, 39.18%; H, 5.25%; N, 11.38%.
Embodiment 2
[0037] The preparation process of the tridentate pyridinium iron complex shown in formula (B) prepared in this example is as follows:
[0038] The 25mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl 2 and tridentate pyridine imine ligand substituted by diethylaminoethyl (1.5 mmol) (structural formula LA), and stirred at room temperature for 24 h. After the reaction, the dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain a light blue solid with the structural formula:
[0039]
[0040] Mass Spectrometry: C 12 h 19 Cl 2 FeN 3 [M-Cl] + : Theoretical value: 296.0611; measured value: 296.0605.
[0041] Elemental Analysis: C 12 h 19 Cl 2 FeN 3 : Theoretical value: C, 43.41%; H, 5.77%; N, 12.66%; Measured value: C, 43.45%; H, 5.75%; N, 12.62%.
Embodiment 3
[0043] The preparation process of the tridentate pyridineimine iron complex shown in formula (C) prepared in this example is as follows:
[0044] The 10mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, anhydrous FeCl 3 and methoxyethyl-substituted tridentate pyridine imine ligand (1.5 mmol) (structural formula LB), and stirred at room temperature for 48 h. After the reaction, the dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain a light red solid, structural formula:
[0045]
[0046] Mass Spectrometry: C 9 h 12 Cl 3 FeN 2 O[M-Cl] + : Theoretical value: 289.9671; measured value: 289.9668.
[0047] Elemental Analysis: C 9 h 12 Cl 3 FeN 2 O: Theoretical value: C, 33.12%; H, 3.71%; N, 8.58%; measured value: C, 33.15%; H, 3.65%; N, 8.61%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com