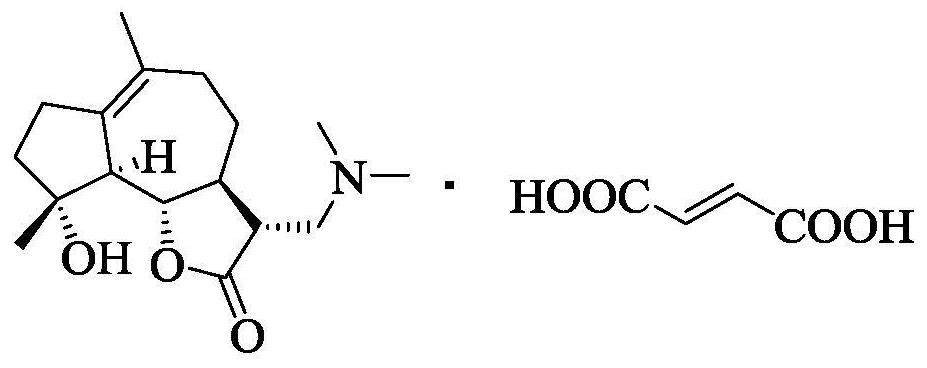Lactone-containing derivative, its pharmaceutical composition and its preparation method and use
A technology of derivatives and compositions, applied in the field of preparing anti-tumor and anti-inflammatory immune drugs, which can solve problems such as drug resistance and insensitivity of tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 Milicolactone (MCL)
[0065]
[0066] Add 80ml of dichloromethane and p-toluenesulfonic acid (2.0mmol) into a 200ml three-necked flask, add dropwise a solution of parthenolide (PTL, 50mmol) and 18ml of dichloromethane under stirring, and continue to Stir overnight. After TLC confirms complete conversion of the PTL starting material, use NaHCO 3 (0.35g) and 3.5ml of water to prepare a solution, add the reaction solution and stir for 1 hour, during the stirring process, the reaction solution gradually fades from purple to yellow, separate the water phase, wash the organic phase twice with saturated brine, and concentrate to obtain a yellow solid , recrystallized with acetone and cooled at low temperature to obtain light yellow solid MCL (82%).
[0067] 1 H NMR (400MHz, Chloroform-d)δ6.22(s,1H,H-13),5.50(s,1H,H-13),3.82(t,1H,H-12),2.75-2.64(m, 2H,H-3,H-11),2.62(s,1H,OH),2.50-1.25(m,8H,),1.69(s,3H,CH3),1.33(s,3H,CH3).
Embodiment 2
[0068] Example 2 5-Hydroxymimicrolactone (5-OH MCL)
[0069]
[0070] Michelin lactone (MCL) (1.0mmol), SeO 2 (0.3mmol), tert-butyl hydroperoxide TBHP (2.0mmol), 30ml dichloromethane were placed in a 100ml round bottom flask, and refluxed for 3 hours. After filtration through celite, the filtrate was concentrated and separated by column chromatography (eluent) to obtain a yellow solid (52.6%).
[0071] 1 H NMR (400MHz, Chloroform-d): δ6.22(s,1H,H-13),5.52(s,1H,H-13),4.33(s,1H,H-5),3.90-3.82(m ,1H,H-12),3.37(t,J=9.5Hz,1H,H-3),2.91(d,J=12.8Hz,1H,H-11),2.67-1.58(m,11H),1.29 (s,3H, H-15).
Embodiment 3
[0072] Example 3 5-oxo michelactone
[0073]
[0074] Put 5-hydroxymiglianolide (1.0mmol), Dess-Martin reagent (0.5mmol), sodium bicarbonate (10mmol), and 30ml of dichloromethane in a 100ml round bottom flask, and stir at room temperature for 3 hours. After the raw material reaction was completed, quench the reaction with saturated sodium bisulfite solution, add 30ml methylene dichloride, wash the organic phase with water and saturated sodium chloride solution respectively, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography (eluent ) gave a white solid (93.6%).
[0075] 1 H NMR(400MHz,Chloroform-d)δ6.34(s,1H,H-13),5.61(s,1H,H-13),4.28(t,J=10.0Hz,1H,H-12),3.16 -3.12(m,1H,H-3),3.00(d,J=10.9Hz,1H,H-11),2.76-1.60(m,10H),1.33(s,3H,H-15).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com