Method for removing nitrate from nickel copper cobalt sulfate solution
A sulfuric acid solution and sulfate technology, applied in nickel sulfate and other directions, can solve the problems such as difficulty in removing nitrate, and achieve the effects of saving equipment investment, low production cost, and good stripping effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The composition of the pre-extraction solution is shown in Table 1.
[0025]
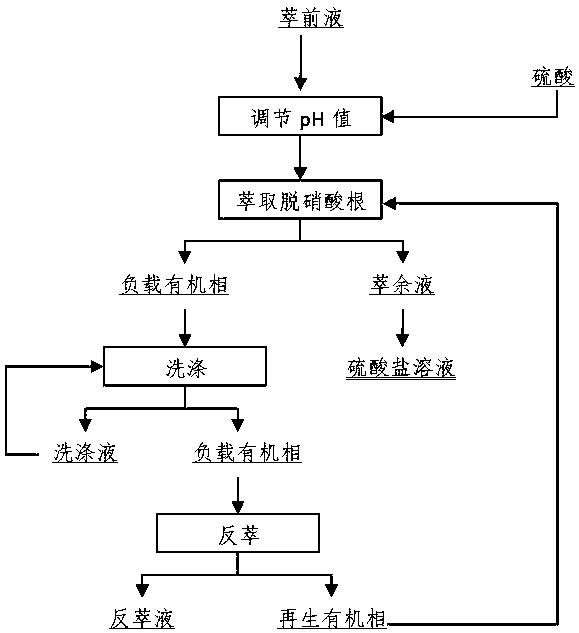
[0026] The method of removing nitrate is as follows:
[0027] A, adjust the pH value: add sulfuric acid solution to adjust the pH value of the pre-extract solution to 1.0;
[0028] B. Extraction and removal of nitrate ions: use the extraction organic phase for extraction, the volume concentration of N235 in the extraction organic phase is 10%, the volume concentration of isooctyl alcohol is 5%, and the volume concentration of sulfonated kerosene is 85%. The ratio O / A is 1:1, single-stage countercurrent extraction is carried out, the phase mixing time is 2min, and the phase separation time is 5min. After extraction, a loaded organic phase containing nitrate ions and a raffinate containing sulfate are obtained respectively. The raffinate Composition is as shown in table 2;
[0029]
[0030] C. Washing: use sulfuric acid to wash the loaded organic phase, the concentration of sulfuric aci...
Embodiment 2
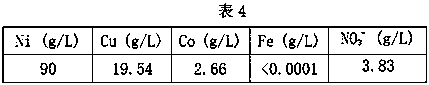
[0034] The composition of the pre-extraction solution is shown in Table 4.
[0035]
[0036] The method of removing nitrate is as follows:
[0037] A, adjust the pH value: add sulfuric acid solution to adjust the pH value of the pre-extract solution to 1.5;
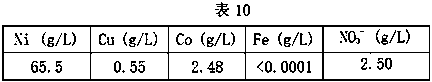
[0038] B. Extraction and removal of nitrate ions: use the extraction organic phase for extraction, the volume concentration of N235 in the extraction organic phase is 15%, the volume concentration of isooctyl alcohol is 20%, and the volume concentration of sulfonated kerosene is 65%. Ratio O / A is 1.5:1, carries out 2 stages of countercurrent extraction, phase mixing time is 3min, phase separation time is 7min, obtains the loaded organic phase containing nitrate ion and the raffinate containing sulfate respectively after extraction, raffinate Composition is as shown in table 5;
[0039]
[0040] C. Washing: Use sulfuric acid to wash the loaded organic phase, the concentration of sulfuric acid for washing is 0.5mol / ...
Embodiment 3
[0044] The composition of the pre-extraction solution is shown in Table 7.
[0045]
[0046] The method of removing nitrate is as follows:
[0047] A, adjust the pH value: add sulfuric acid solution to adjust the pH value of the pre-extraction solution to 1.75;
[0048] B. Extraction and removal of nitrate ions: use the extraction organic phase for extraction, the volume concentration of N235 in the extraction organic phase is 25%, the volume concentration of isooctyl alcohol is 30%, and the volume concentration of sulfonated kerosene is 45%. Ratio O / A is 1.5:1, carry out 2 stages of countercurrent extraction, phase mixing time is 4min, phase separation time is 10min, after extraction, respectively obtain the loaded organic phase containing nitrate ions and the raffinate containing sulfate, the raffinate Composition is as shown in table 8;
[0049]
[0050] C. Washing: Use sulfuric acid to wash the loaded organic phase, the concentration of sulfuric acid for washing is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com