Method for preparing PCA3 (prostate cancer antigen 3) quantitative detection standard
A quantitative detection and standard technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as low specificity and negative puncture, and achieve the effect of high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The method for preparing PCA3 quantitative detection standard substance of the present embodiment, comprises the following steps:
[0027] Step 1: Synthesizing PCA3 DNA, and the synthesized sequence is coded as PCA3-1;
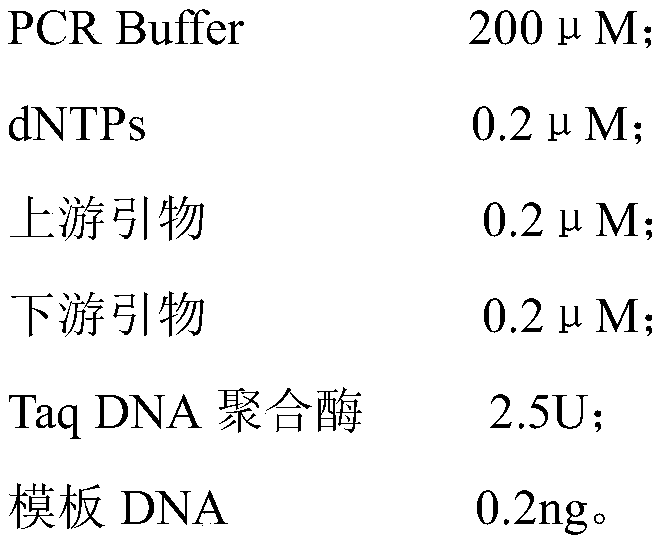
[0028] Step 2: PCR amplification; define the upstream and downstream primer codes as PCA3-F and PCA3-R respectively, and the 25ul reaction system is:
[0029]
[0030] The PCR amplification program specifically includes the following steps:
[0031] S1, after configuring the components required for the PCR reaction, preheat the PCR instrument at 95°C for 5 minutes to fully denature the template DNA, and then enter the amplification cycle;
[0032] S2, Amplification cycle: In each cycle, first keep at 95°C for 10s to denature the template, then lower the temperature to 55°C for 10s to fully anneal the primers to the template, and finally keep at 72°C for 30s to make the primers on the template Up extension, DNA synthesis, complete a cycle, repeat th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com