Tetrameric macrocyclic trinuclear iron complex containing sulfur-sulfur bonds and in-situ synthesis method thereof
An iron complex, in-situ synthesis technology, applied in organic chemical methods, drug combinations, topical antibacterial agents, etc., to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] A method for in-situ synthesis of a tetrameric macrocyclic trinuclear iron complex containing sulfur-sulfur bonds, comprising the steps of:
[0017] (1) Weigh 0.0400g 3,3’-bis(5-mercapto-1,2,4-triazole) (H 4 L) and 0.0808g iron nitrate nonahydrate Fe(NO 3 ) 3 9H 2 O, mixed in 8 mL dimethylformamide (DMF), and the mixture was stirred and reacted at room temperature for 6 h;
[0018] (2) The above mixture was filtered, and the filtrate was placed in a 50 mL beaker, and naturally volatilized at room temperature to obtain single crystal grade black flaky crystals.
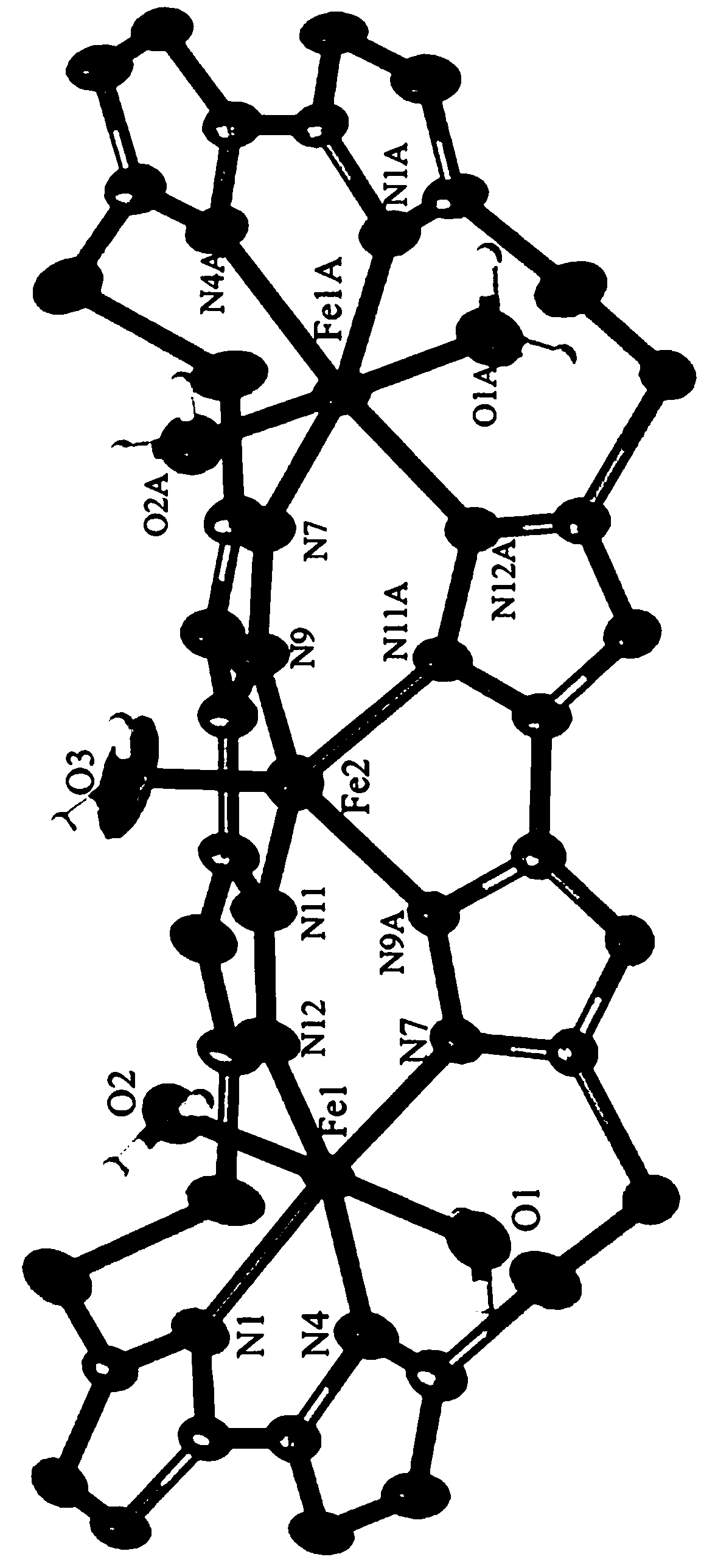
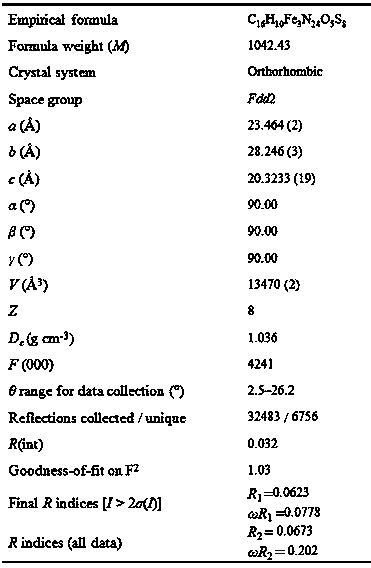
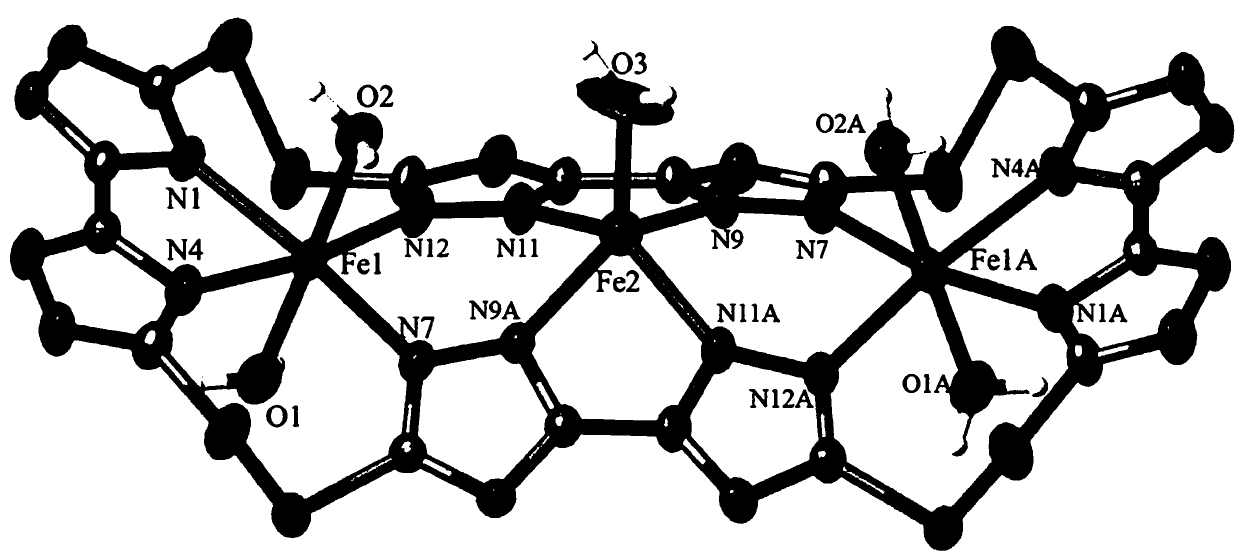
[0019] see figure 1 , a schematic diagram of the molecular structure of the four polycyclic macrocyclic trinuclear iron complexes containing sulfur-sulfur bonds in the embodiment. It can be seen clearly from the figure that the complex is a trinuclear iron structure composed of three iron ions [2 Fe ( Ⅲ) and one Fe (II)], one macrocyclic ligand and five waters. Among them, Fe1 coordinates with the four N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com