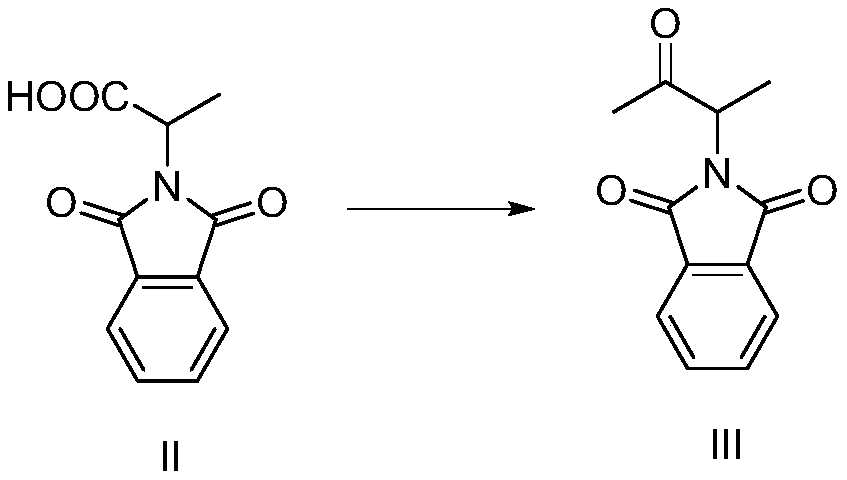
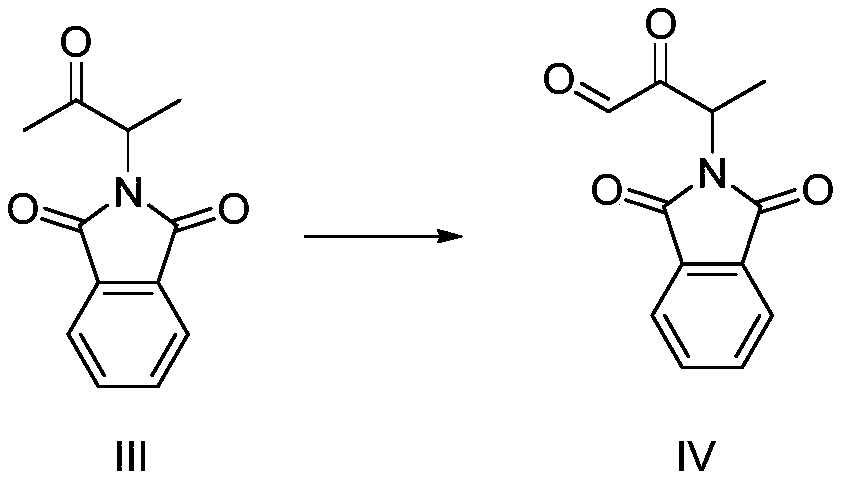
Preparation method of 3-phthalimide-2-oxy-n-butyraldehyde thiosemicarbazide
A technology of n-butyraldehyde bisthiosemicarbazide and phthalimide, which is applied in the direction of organic chemistry, can solve the problems of difficult filtration, cumbersome operation, and long reaction route, so as to improve efficiency, reduce operating cost, and facilitate operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
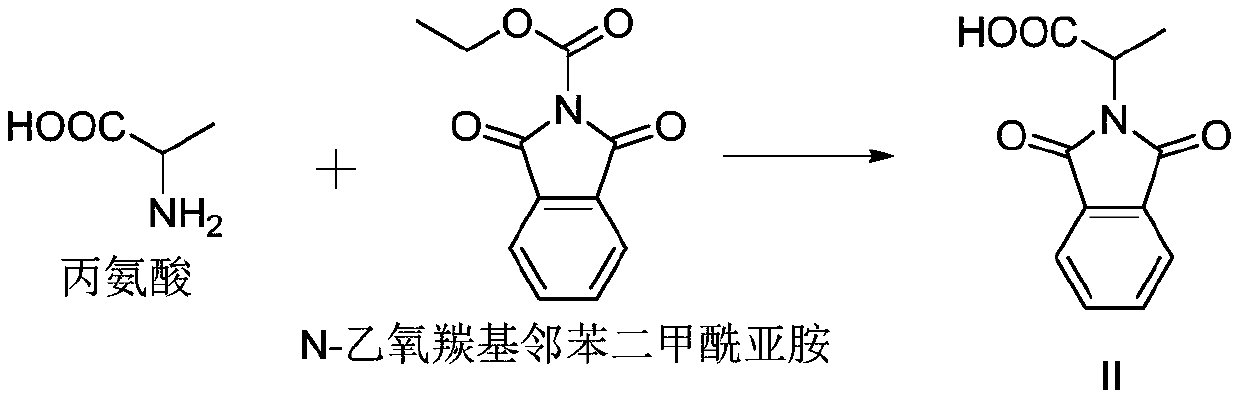
[0039] Embodiment 1: preparation compound II
[0040] Take N-ethoxycarbonylphthalimide (21.9g, 0.1mol), sodium carbonate (10.6g, 0.1mol) and 100mL water in a 250mL single-necked bottle, add dropwise alanine (8.9g, 0.1mol ) in 30 mL aqueous solution, stirred at room temperature for 2 h, added dropwise 2M hydrochloric acid to adjust the pH value to 2-3, filtered and dried to obtain 18 g of compound II. Yield 82%.
[0041] 1 HNMRδ1.71(s,3H),5.02(q,1H),7.69-7.75(m,2H),7.82-7.88(m,2H).MS(ESI)m / z 218.2[M-H] -
[0042] Table 1: Screening of Reaction Conditions for Preparation of Compound II
[0043]
Embodiment 2
[0044] Embodiment 2: preparation compound II
[0045] Take N-ethoxycarbonylphthalimide (21.9g, 0.1mol), sodium carbonate (10.6g, 0.1mol) and 100mL water in a 250mL single-necked bottle, add alanine (8.9 g, 0.1mol) in 30mL aqueous solution, after dripping, naturally rise to room temperature and stir for 2h, add dropwise 2M hydrochloric acid to adjust the pH value to 2-3, filter and dry to obtain 17g of compound II with a yield of 78%.
Embodiment 3
[0046] Embodiment 3: preparation compound II
[0047] Take N-ethoxycarbonylphthalimide (21.9g, 0.1mol), sodium carbonate (12.7g, 0.12mol) and 100mL water in a 250mL single-necked bottle, add alanine (8.9g, 0.1mol) dropwise ) in 30 mL aqueous solution, stirred at room temperature for 2 h, added dropwise 2M hydrochloric acid to adjust the pH value to 2-3, filtered and dried to obtain 17.5 g of compound II with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com