Method for producing endothelial cells
A technology of endothelial cells and endothelial progenitor cells, applied in the direction of vascular endothelial cells, germ cells, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Differentiation of iPS cells into vascular endothelial cells
[0122] Human iPS cells (ChiPSC12 strain) (Cellartis) were cultured in DEF-CS medium (Cellartis) according to the instructions attached to the medium.
[0123] In order to effectively induce the differentiation of mesoderm cells, iPS cells were coated with Matrigel according to the following procedure (Matrigel sandwich method). First, TrypLE (trademark) Select (Life technologies) was added to subcultured iPS cells, and the cells were incubated at 37°C for 3-7 minutes. Collect the separated cells as single cells by pipetting, and count the number of cells. Next, divide the cells into 6 x 10 4 Cells / cm 2 The density was inoculated into a culture vessel coated with Matrigel (trademark) (Corning) and cultured in DEF-CS medium for 2-3 days. After that, the medium was replaced with DEF-CS medium supplemented with Matrigel diluted to 1:60, and the cells were cultured for a further 16-24 hours to coat the up...
Embodiment 2
[0134] Example 2: Freezing and thawing of vascular endothelial cells
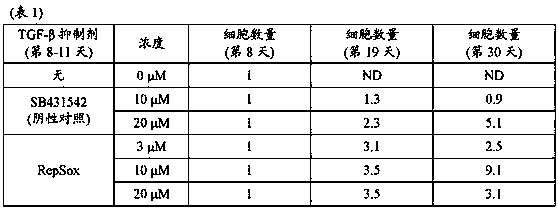
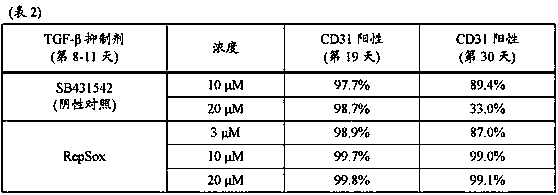
[0135] The induction of vascular endothelial cells was performed according to the method described in Example 1 using the medium supplemented with 20 μM RepSox, and the cells were collected from day 18-22. Wash the collected cells and suspend them in Stem Cell Banker (TAKARABIO INC.) so that the cell concentration is 3 x 10 6 / mL, and dispense 1 mL of each suspension in vials. The vial was placed in a refrigerated container (BICELL, Nihon Freezer Co., Ltd.), and the cells were subjected to slow freezing in a freezer storage chamber maintained at a temperature of -80°C. After that, each vial was transferred to liquid nitrogen and stored for 3 days.
[0136] The frozen cells were warmed in a water bath warmed to 37°C for 2 minutes and 30 seconds to thaw the cells. The cell viability was measured by using a part of the cells, and the result was that the cell viability was 87%. The entire amount of the remaining ...
Embodiment 3
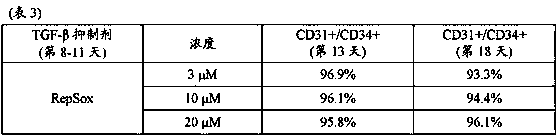
[0138] Example 3: Measurement of CD31+ / CD34+ cells
[0139] According to the instructions attached to the medium, human iPS cells (ChiPSC12 strain) were cultured in DEF-CS medium.
[0140] TrypLE (trademark) Select was added to iPS cells that had been subcultured, and the cells were incubated at 37°C for 3-7 minutes. Collect the separated cells as single cells by pipetting, and count the number of cells. Next, divide the cells into 6x 10 4 Cells / cm 2 The density was inoculated into a culture vessel coated with Matrigel (trademark), and cultured in DEF-CS medium for 2-3 days. After that, the medium was replaced with DEF-CS medium supplemented with Matrigel diluted to 1:60, and the cells were cultured for a further 16-24 hours to coat the upper layer of the cells with Matrigel.
[0141] The obtained Matrigel-coated iPS cells were differentiated into mesoderm cells according to the following procedure. First, the medium of Matrigel-coated iPS cells was replaced with RPMI 1640 medium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com