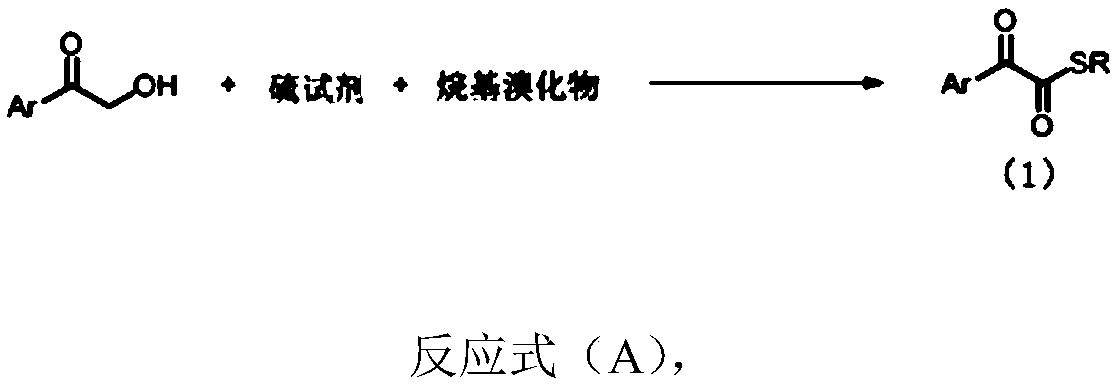
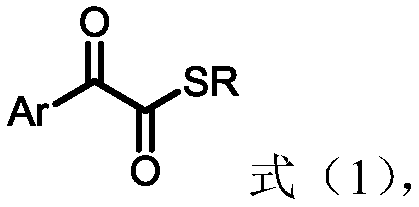
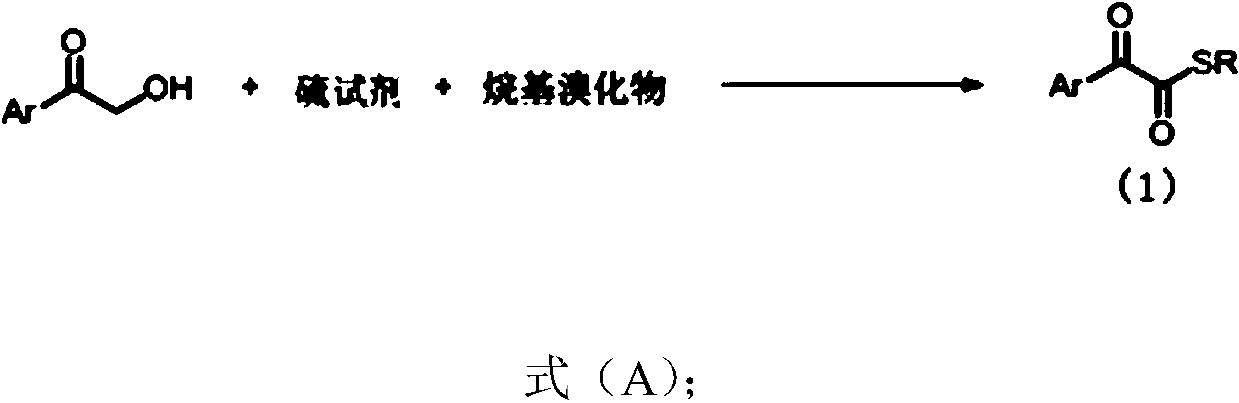
1,2-dicarbonyl compounds and synthesis method thereof
A synthesis method and compound technology, applied in the first field, can solve problems such as instability and many side reactions, and achieve the effects of good yield, simple synthesis method, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Compound 2a:
[0044]
[0045] Under nitrogen protection, α-hydroxyacetophenone (0.5mmol), S 8 (64.2 mg, 2.0 mmol, 4.0 equiv), KHCO 3 (100mg, 1.0mmol, 2.0equiv), TBAB (32.3mg, 0.1mmol, 20mol%) were added to the reaction tube with the magneton placed, evacuated for three times and then added H 2 O (4mmol, 20equiv) and solvent CPME (4mL), the reaction system was heated to 90 ° C for 10 hours, and after the conversion of α-hydroxyacetophenone was detected by spotting, add benzyl bromide (0.75mmol, 1.5equiv) to the system , continued to react for 2 hours, lowered to room temperature, added water to the system to dilute, then added ethyl acetate (10mL*3) to extract, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a yellow solid 2a (90 %). 1 H NMR (400MHz, CDCl 3)δ8.14(d, J=8.2Hz, 2H), 7.65(t, J=7.4Hz, 1H), 7.49(t, J=7.8Hz, 2H), 7.40-7.27(m, 5H), 4.28( s, 2H). 13 C NMR (100MHz, CDCl 3 )δ191.9...
Embodiment 2
[0047] Synthesis of compound 2a':
[0048]
[0049] Under nitrogen protection, α-hydroxyacetophenone (3.0mmol), S 8 (12.0 mmol, 4.0 equiv), KHCO 3 (6.0mmol, 2.0equiv), TBAB (0.6mmol, 20mol%) was added to the reaction tube with the magneton placed, and after evacuating nitrogen for three times, add H 2 O (60mmol, 20equiv) and solvent CPME (20mL), the reaction system was heated to 90 ° C for 10 hours, and after the spot plate detection of α-hydroxyacetophenone was completely converted, ethyl bromide (6.0mmol, 2equiv) was added to the system , continued to react for 2 hours, lowered to room temperature, added water to the system to dilute, then added ethyl acetate (30mL*3) to extract, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain yellow oil 2a' (81%). 1 H NMR (400MHz, CDCl 3 )δ8.16-8.08(m, 2H), 7.68-7.62(m, 1H), 7.54-7.47(m, 2H), 3.06(q, J=7.4Hz, 2H), 1.37(t, J=7.4Hz , 3H). 13 C NMR (100MHz, CDCl 3 )δ193.0,...
Embodiment 3
[0051] Synthesis of compound 2b:
[0052]
[0053] Under nitrogen protection, α-hydroxyl 4-methylacetophenone (0.5mmol), S 8 (64.2 mg, 2.0 mmol, 4.0 equiv), KHCO 3 (100mg, 1.0mmol, 2.0equiv), TBAB (32.3mg, 0.1mmol, 20mol%) was added to the reaction tube with the magneton placed, and after evacuating nitrogen for three times, add H 2 O (4mmol, 20 equiv) and solvent CPME (4mL), the reaction system was heated to 90 ° C for 10 hours, after spot plate detection of α-hydroxyacetophenone complete conversion, add benzyl bromide (0.75mmol, 1.5equiv ), continued to react for 2 hours, lowered to room temperature, added water to the system to dilute, then added ethyl acetate (10mL*3) for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a yellow solid 2b ( 72%). 1 H NMR (400 MHz, CDCl 3 )δ8.06(d, J=8.3Hz, 2H), 7.41-7.27(m, 7H), 4.28(s, 2H), 2.44(s, 3H). 13 CNMR (100MHz, CDCl 3 )δ192.2, 185.4, 146.2, 136.4, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com