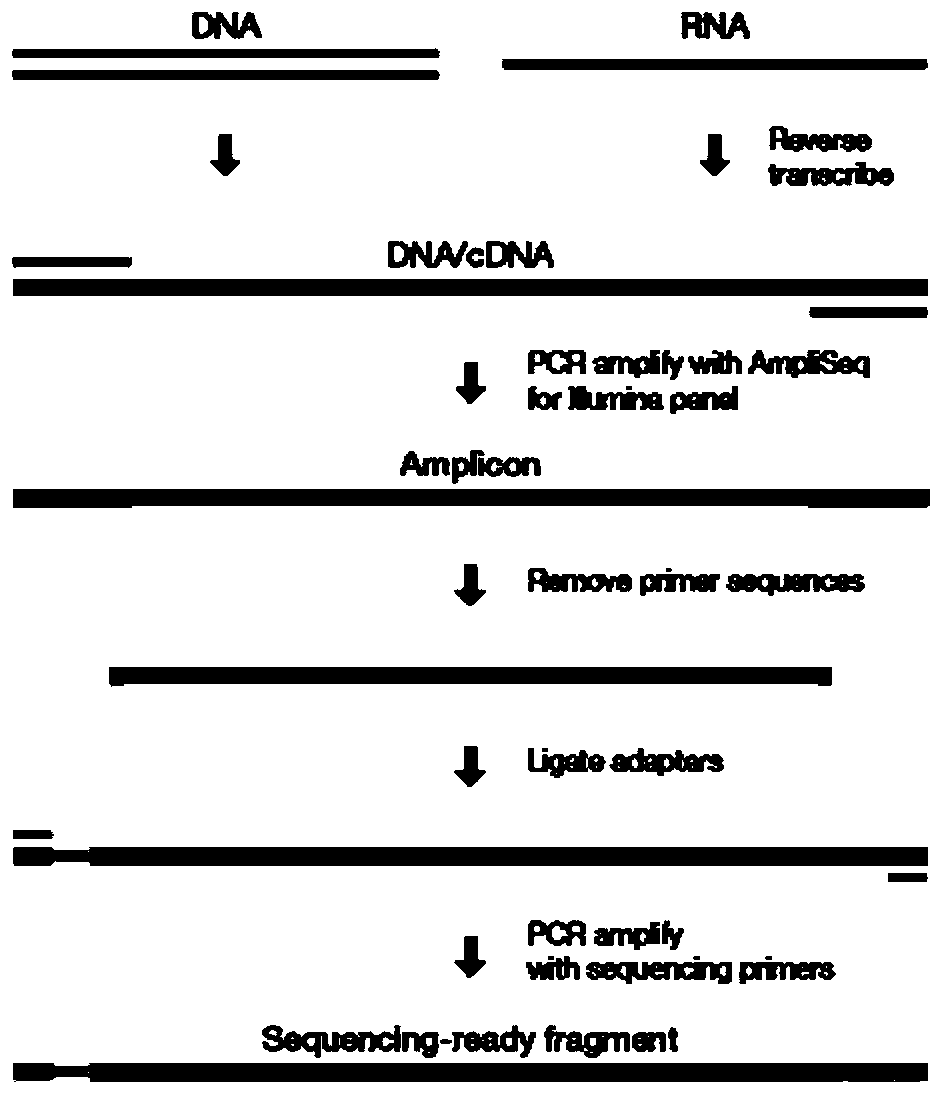
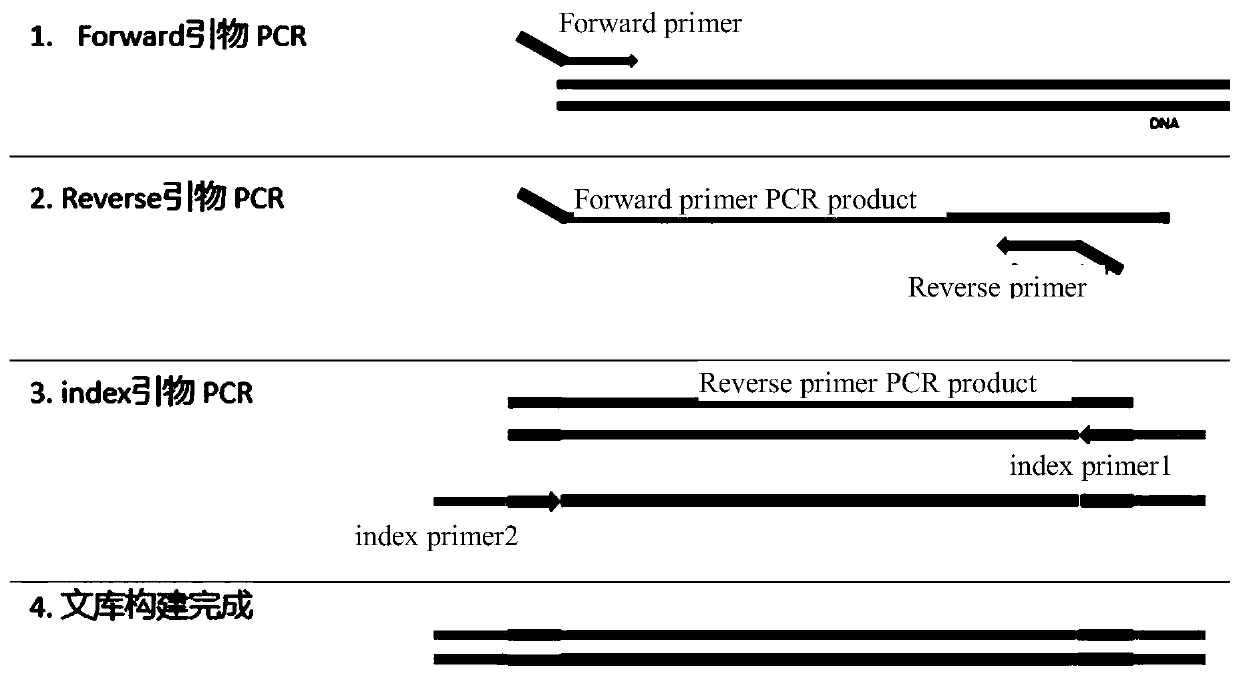
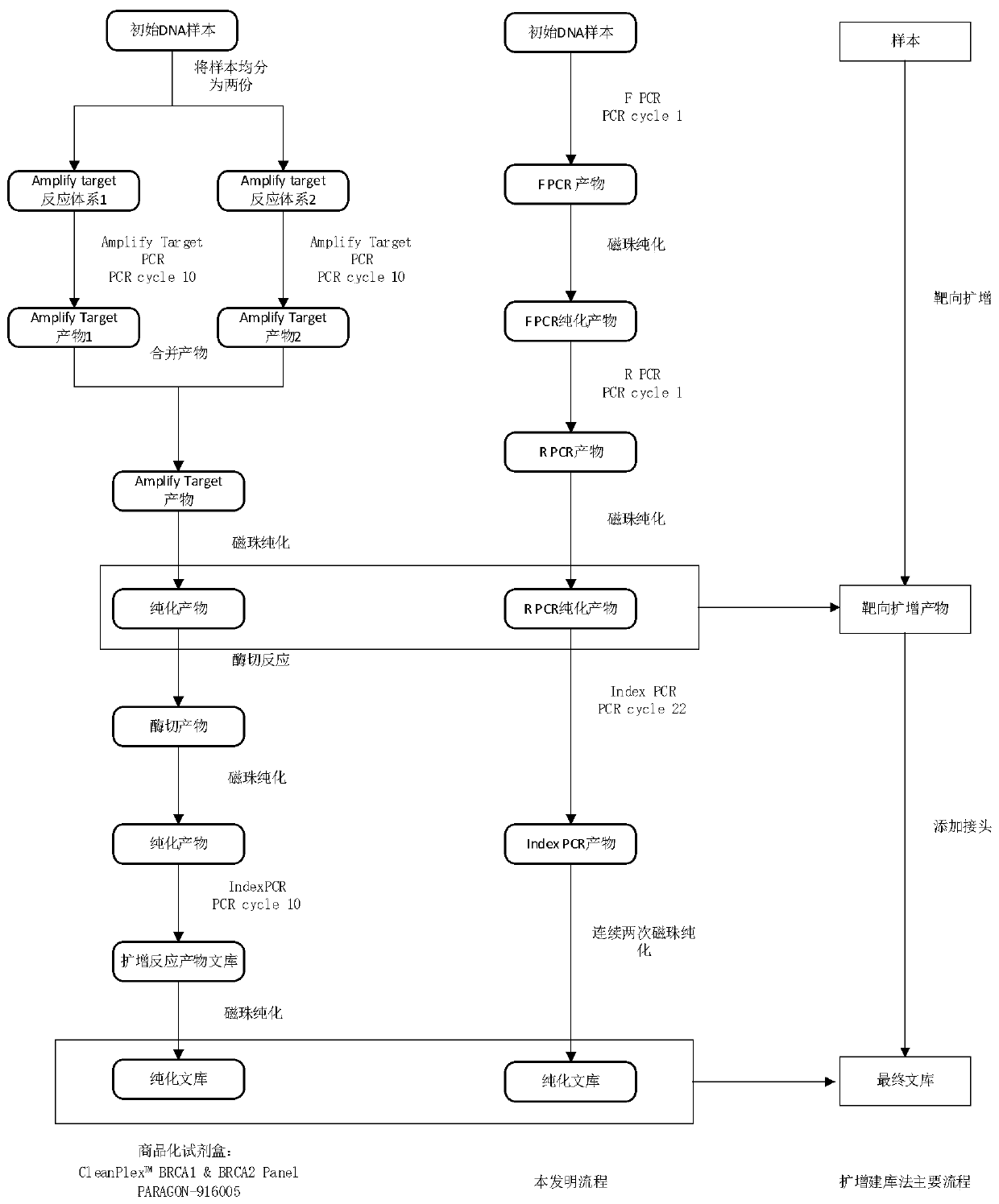
Automated reagent kit for constructing BRCA1/2 gene variation detection library
A kit and gene technology, applied in the field of building BRCA1/2 gene variation detection library, can solve the problems of difficulty in ensuring the consistency of experimental operation, increasing the cumbersome operation, detecting sample demand, and difficulty in realizing full automation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0050] BRCA1 / 2 library construction
[0051] 1 Main reagents and instruments
[0052]
[0053]
[0054] 2 experimental operation
[0055] 2.1 Forward Primer-PCR
[0056] 2.1.1 Using Hot-Start DNA Polymerase in a Ready-to-Use MasterMix, in a PCR tube, configure the F-PCR mix as follows:
[0057]
[0058] 2.1.2 Slightly vortex and mix well, then centrifuge briefly, place the PCR tube in the PCR instrument, and run the following program:
[0059]
[0060] 2.2 F primer PCR product purification
[0061] 2.2.1 Take the agencourt ampu0re XP-PCR purification beads out of the refrigerator at 4°C in advance, and equilibrate at room temperature for 30 minutes;
[0062] 2.2.2 Vortex the ampure beads after equilibrating at room temperature, take 48ul ampure beads (1.2X) to a new 1.5mL centrifuge tube, and transfer all 40ul PCR products from the previous step into it;
[0063] 2.2.3 Vortex the PCR product and magnetic beads, and incubate at room temperature for 5 minutes;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com