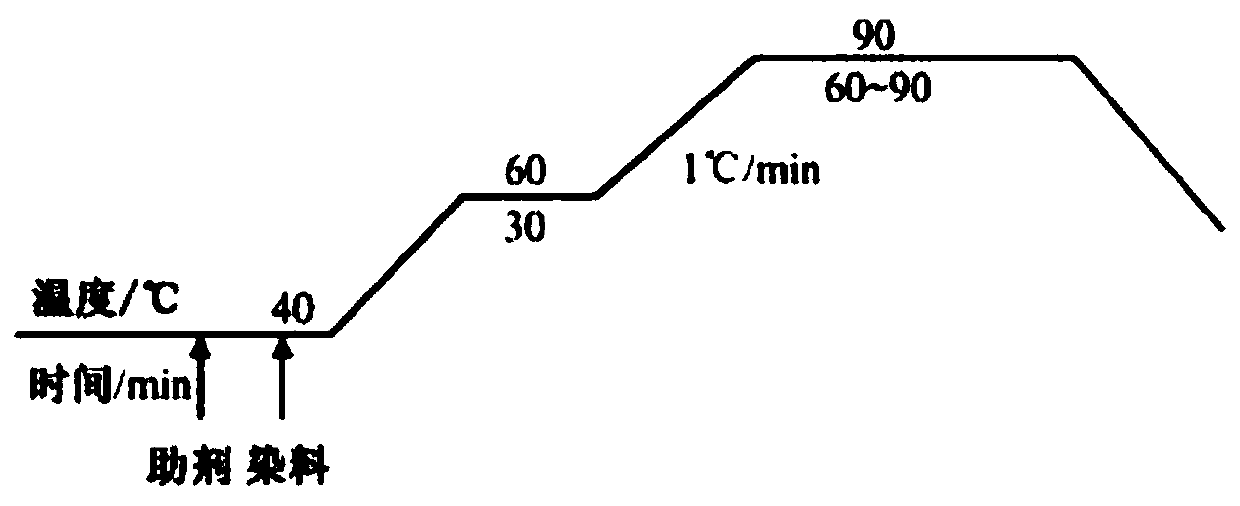
Disazo light-fast acid dye containing h-acid structure and preparation method thereof
An acid dye, disazo technology, used in the chemical industry, can solve the problems of poor light fastness, large gap, color cast, etc., and achieve the effect of improving light fastness, high light fastness and excellent performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
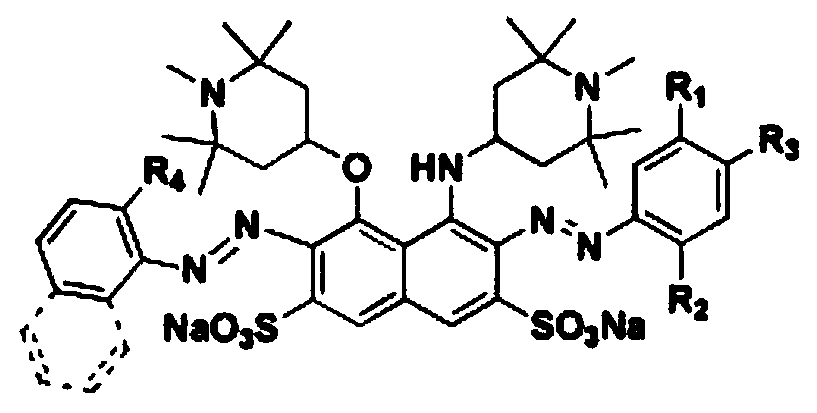
[0039] Embodiment 1, a kind of preparation method that contains H acid structure disazo type sunfast acid dye (black dye), carries out following steps successively:
[0040] 1), the chlorination reaction of pentamethylpiperidinol:
[0041] Add 17.4mL (0.24mol) of thionyl chloride to a 100mL three-necked flask, and dissolve 10.3g (0.06mol) of 1,2,2,6,6-pentamethylpiperidinol in batches under stirring Slowly (the purpose is to control the temperature not to exceed 60°C) into the above-mentioned three-neck flask, heat up to 80°C, and continue to stir and react for 1.5h. After the reaction is over, transfer the reaction solution to a 250mL beaker, add 100mL of absolute ethanol to the beaker, stir well, and slowly add triethylamine dropwise to it under ice bath conditions until the pH of the solution is adjusted to neutral , add 100mL ether to the beaker, a white precipitate appears in the solution, stir evenly until no new precipitate occurs; filter and wash the filter cake twice...
Embodiment 2
[0049] Embodiment 2, a kind of preparation method that contains H acid structure disazo type sunfast acid dye (blue dye), carries out following steps successively:
[0050] 1), the chlorination reaction of pentamethylpiperidinol: with the step 1) of embodiment 1.
[0051] 2), condensation reaction:
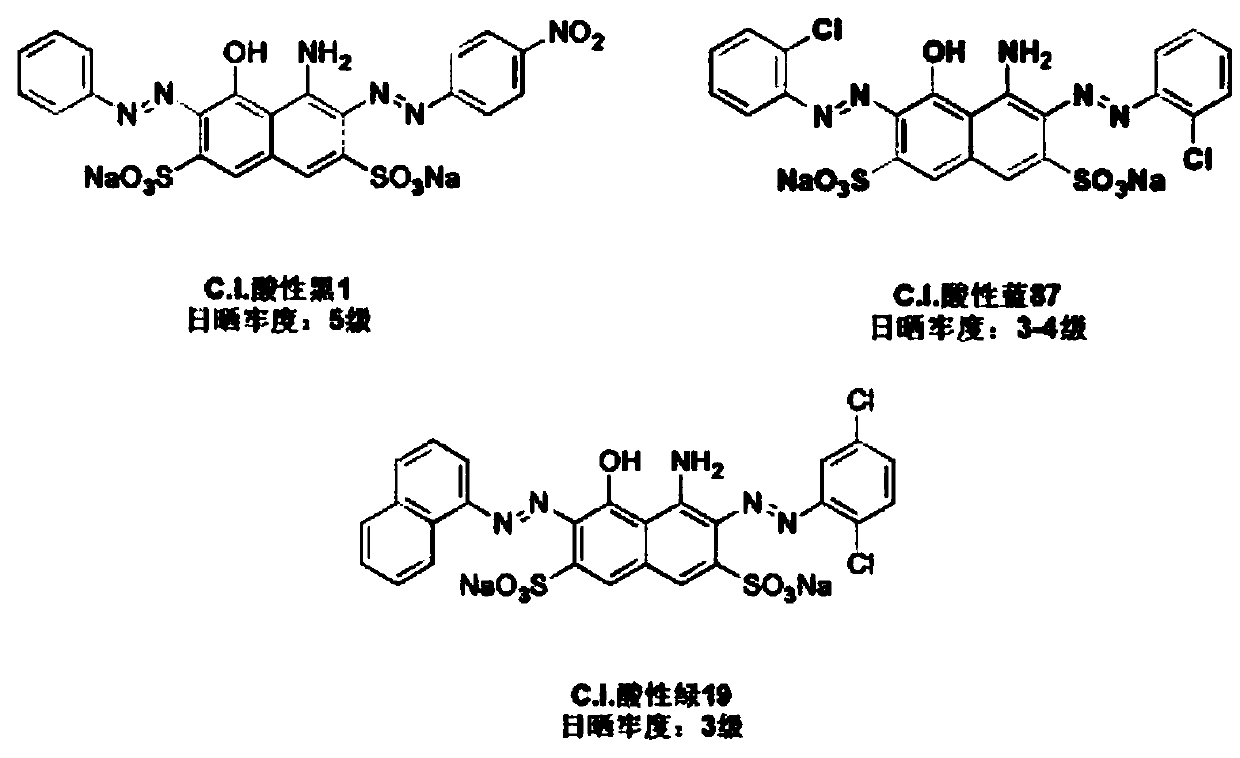
[0052] Weigh 0.02mol NaOH and dissolve it in 30mL deionized water, transfer it to a three-necked flask, add 0.01mol C.I. acid blue 87, stir until completely dissolved, heat up to 46-50°C and react for 2 hours, and the HALS-Cl obtained in step 1) (0.02mol) was added dropwise to the above solution, and after the dropwise addition was completed, the temperature was raised to 91-95°C, and the reaction was continued for 8 hours while stirring.
[0053] After the reaction is completed, solid sodium chloride is added to the reaction product solution until the sodium chloride is saturated, the dye product is precipitated by salt, and the salt-containing dye is obtained by filtration; the...
Embodiment 3
[0057] Embodiment 3, a kind of preparation method that contains H acid structure disazo type sunfast acid dye (green dye), carries out following steps successively:
[0058] 1), the chlorination reaction of pentamethylpiperidinol: with the step 1) of embodiment 1.
[0059] 2), condensation reaction:
[0060] Weigh 0.02mol NaOH and dissolve it in 30mL deionized water, transfer it to a three-necked flask, add 0.01mol C.I. Acid Green 19, stir until completely dissolved, heat up to 46-50°C and react for 2 hours, and the HALS- Cl (0.02mol) was added dropwise to the above solution, and after the dropwise addition was completed, the temperature was raised to 91-95° C., and the reaction was continued for 8 hours while stirring.
[0061] After the reaction is completed, add sodium chloride to the reaction product solution until the sodium chloride is saturated, the dye product is precipitated by salt, and the salt-containing dye is obtained by filtration; the salt in the salt-containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com