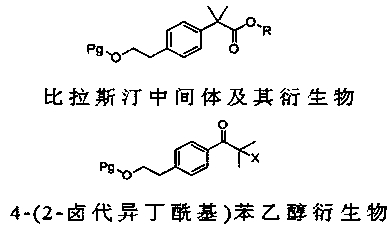
4-(2-halogenated isobutyryl) phenylethanol derivative and preparation method thereof
A technology for halogenating isobutyryl and phenethyl alcohol, applied in the field of 4-phenylethyl alcohol derivatives and preparation thereof, can solve unseen problems and the like, and achieve the effects of low cost, high industrial production and application value, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Preferred examples of the invention will be described in detail below. The examples given are to better explain the content of the invention, and the content of the invention is not limited to the examples. Non-essential improvements and adjustments to the embodiment according to the content of the invention still belong to the scope of the invention.
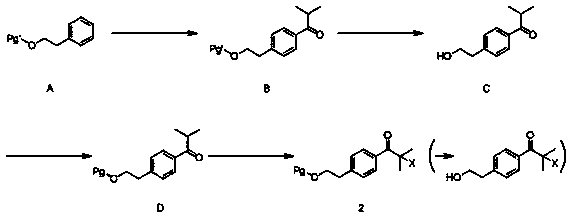
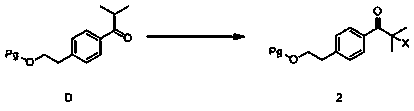
[0029] (1), Synthesis of 4-(2-chloroisobutyryl) phenylethyl alcohol acetate (2) (Pg=CH 3 CO,X=Cl)
[0030]
[0031] In a 500ml three-neck flask, weigh phenylethyl acetate (20.0g, 0.12mol), add 100ml of dichloromethane and stir, cool down to 0°C, add anhydrous aluminum trichloride (32.0g, 0.24mol) and stir, drop Chloroisobutyryl chloride (19.7 g, 0.14 mol) was added, kept at 0°C for 1 hour, returned to room temperature (25°C) and stirred for 4 hours. After the reaction, dilute hydrochloric acid was added to quench the reaction, dichloromethane was added to extract twice, the organic phases were combined, the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com