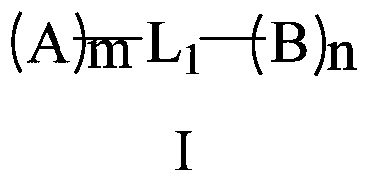Compound, display panel and display device
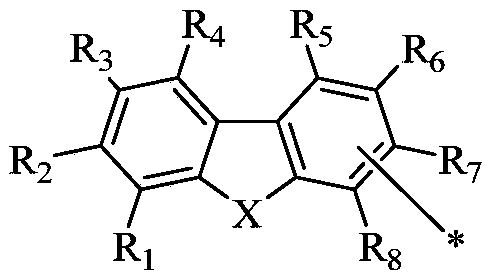
A compound and connection position technology, which is applied in the field of organic electroluminescent materials, can solve the problems of device electron and hole mobility imbalance, device efficiency and life reduction, electron transport performance reduction, etc., to achieve improved ability and high electron transfer efficiency, excellent film stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
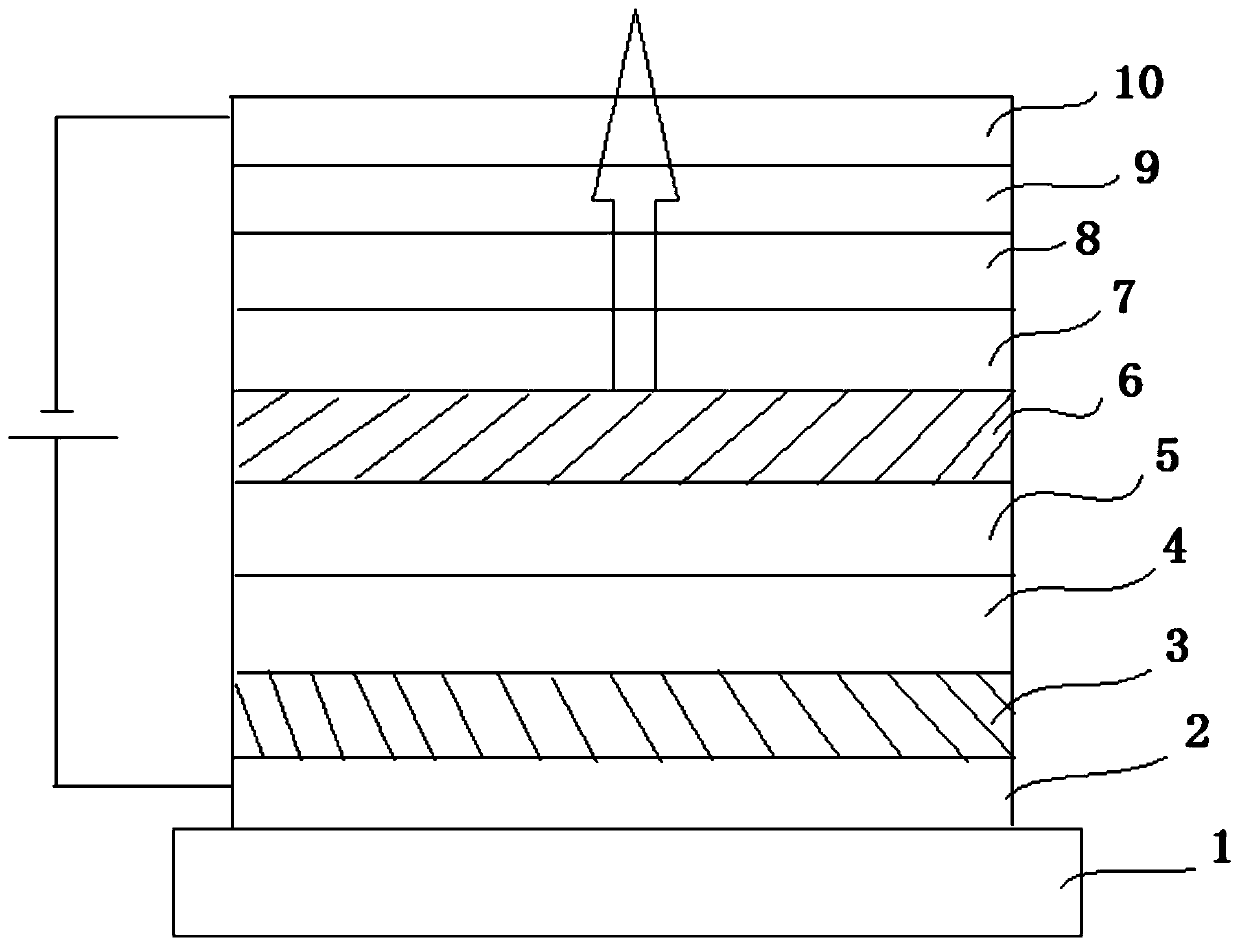
Image
Examples
Embodiment 1
[0117] Synthesis of compound ET01
[0118]
[0119] Nitrogen was passed into a 250mL three-necked flask, 1-bromo-3,5-diiodobenzene (10mmol), 4-phenyl-2-boronic acid ester-quinazoline (10mmol) and Na 2 CO 3 were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed solution, Pd (PPh 3 ) 4 (0.48mmol) was added to the above mixed solution, and the reaction was refluxed under nitrogen atmosphere for 20h. The mixture was then cooled to room temperature and extracted with ethyl acetate. The aqueous layer was further extracted with dichloromethane, and the combined organic layers were washed with brine, MgSO 4 After drying, filtering and concentrating, the residue was recrystallized from dichloromethane and methanol to obtain the product ET01-1;
[0120] Nitrogen was passed into a 250mL three-necked flask, and the intermediate ET01-1 (10mmol), 3-boronate-dibenzofuran (20mmol) and Na 2 CO 3 were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed ...
Embodiment 2
[0123] Synthesis of Compound ET02
[0124]
[0125] Nitrogen was passed into a 250mL three-necked flask, 1-bromo-4-iodobenzene (10mmol), 4-phenyl-2-boronate-quinazoline (10mmol) and Na 2 CO 3were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed solution, Pd (PPh 3 ) 4 (0.48mmol) was added to the above mixed solution, and the reaction was refluxed under nitrogen atmosphere for 20h. The mixture was then cooled to room temperature and extracted with ethyl acetate, the aqueous layer was further extracted with dichloromethane, the combined organic layers were washed with brine, MgSO 4 Dry, filter and concentrate. The residue was recrystallized from dichloromethane and methanol to obtain the product ET02-1;
[0126] Nitrogen was passed into a 250mL three-necked flask, and the intermediate ET02-1 (10mmol), 1-boronate-dibenzofuran (10mmol) and Na 2 CO 3 were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed solution, Pd (PPh 3 ) 4 (0.48mm...
Embodiment 3
[0129] Synthesis of compound ET03
[0130]
[0131] Nitrogen was passed into a 250mL three-necked flask, 4'-bromo-3,5-diiodo-1,1'-biphenyl (10mmol), 4-phenyl-2-boronic acid ester-quinazoline (10mmol) and Na 2 CO 3 were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed solution, Pd (PPh 3 ) 4 (0.48mmol) was added to the above mixed solution, and the reaction was refluxed under nitrogen atmosphere for 20h. The mixture was then cooled to room temperature and extracted with ethyl acetate, the aqueous layer was further extracted with dichloromethane, the combined organic layers were washed with brine, MgSO 4 Dry, filter and concentrate. The residue was recrystallized from dichloromethane and methanol to give the product ET03-1;
[0132] Nitrogen was passed into a 250mL three-necked flask, and the intermediate ET03-1 (10mmol), 3-boronate-dibenzofuran (20mmol) and Na 2 CO 3 were added to toluene / EtOH / H 2 O (75 / 25 / 50mL) solvent, forming a mixed solution, P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com