Novel sorafenib eutectic and preparation method thereof
A technology of sorafenib and co-crystal, applied in the field of drug crystal forms, can solve the problems of low bioavailability, side effects, large oral dose, etc., achieve loose storage and transportation conditions, reduce production costs, and simple and stable preparation processes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
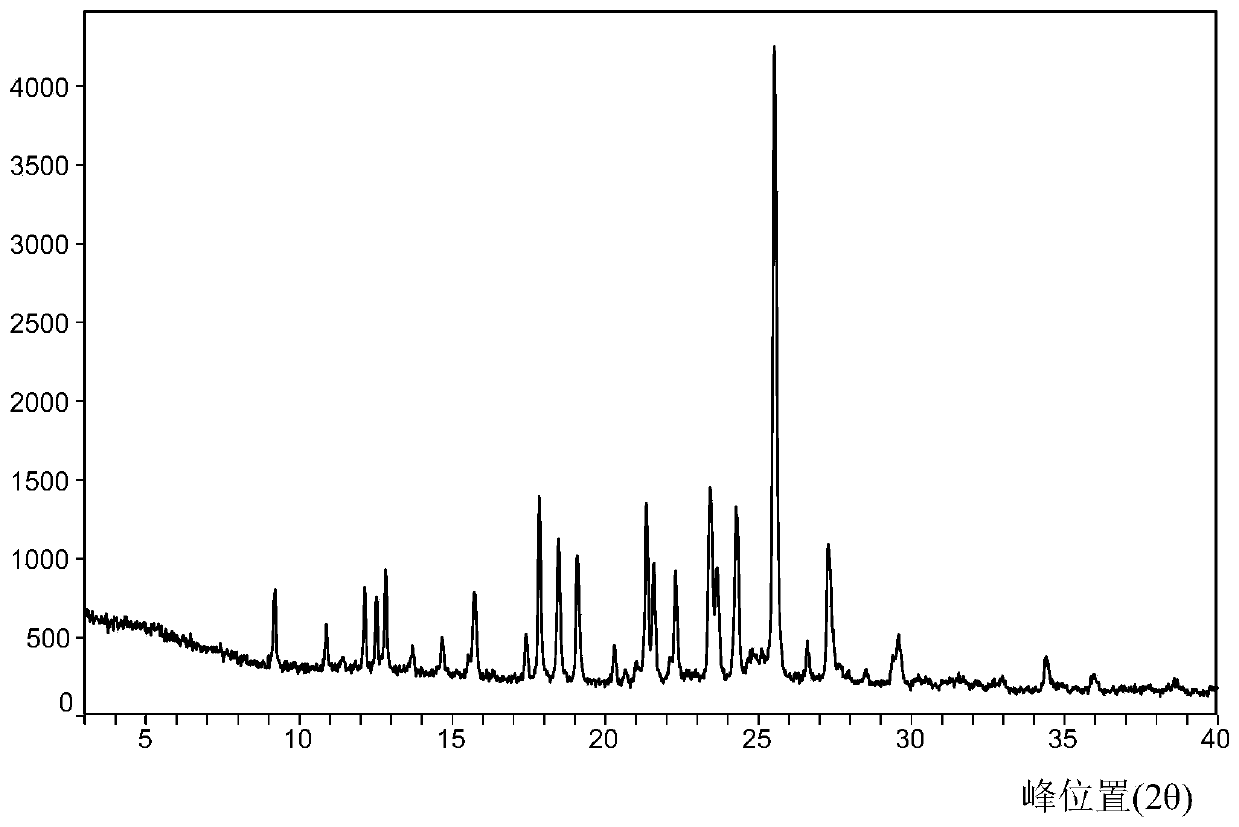
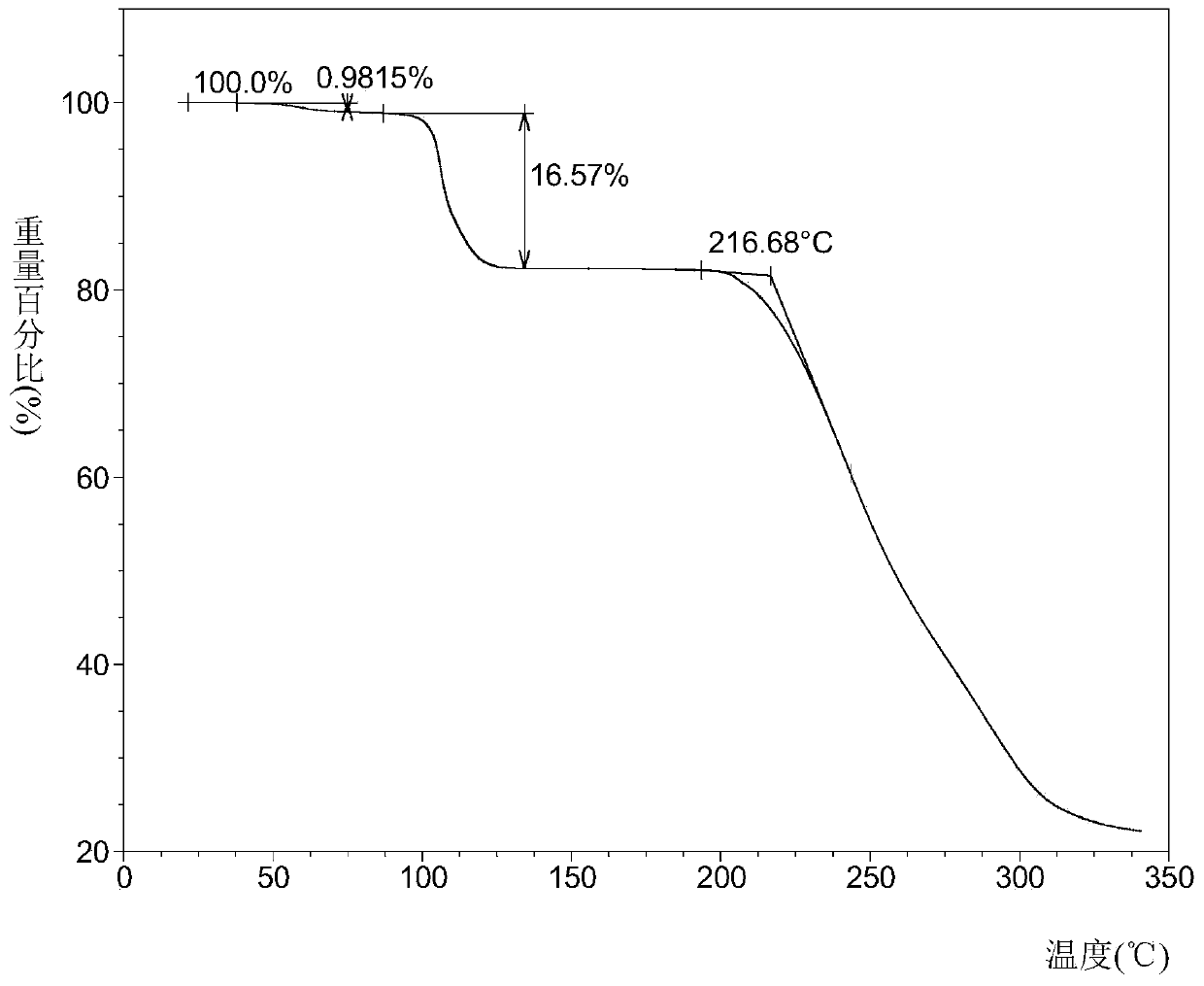
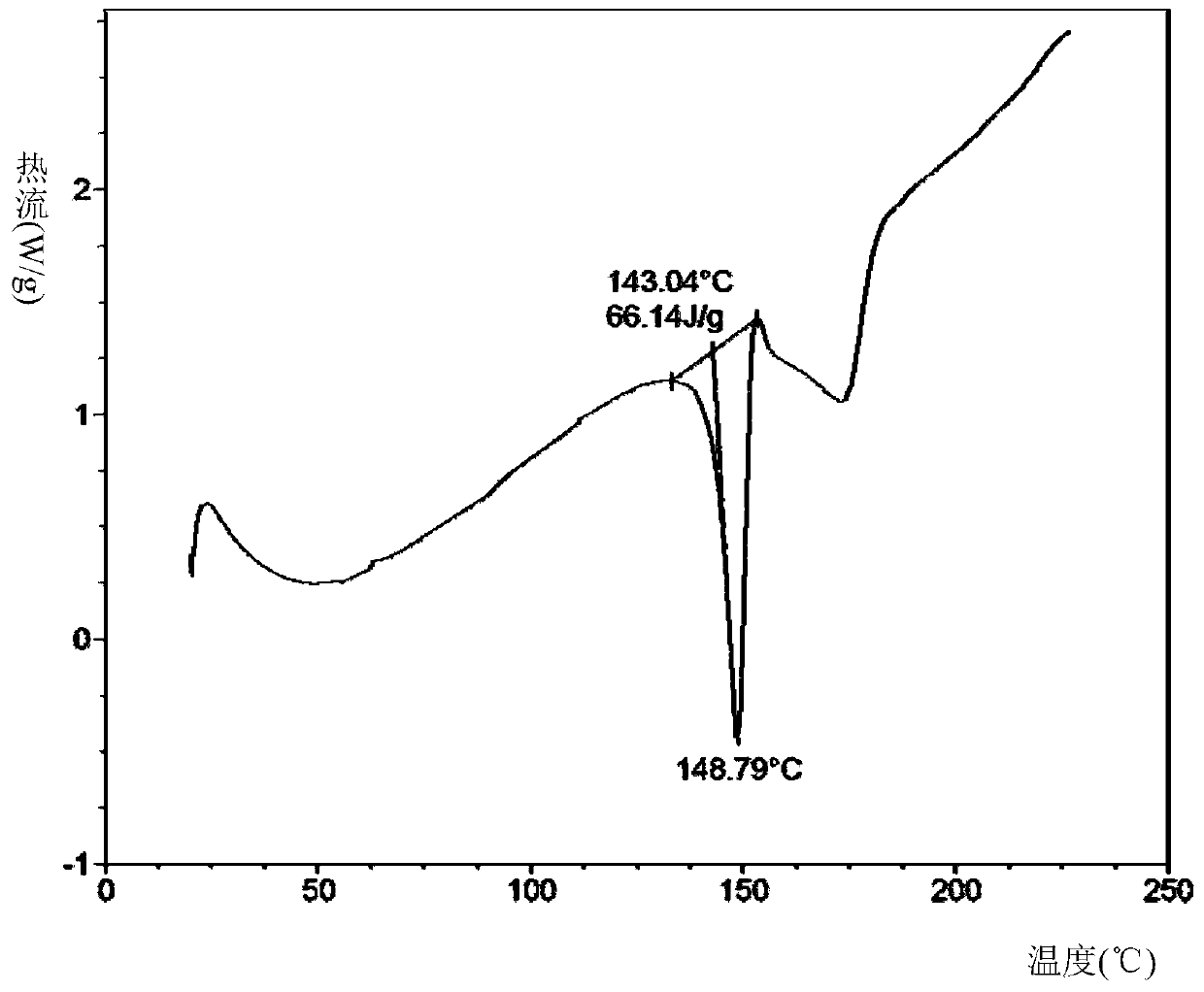
[0034] Take 200 mg of sorafenib free base and 41 mg of 2-aminopyrimidine, mix them, add 8 mL of tetrahydrofuran to dissolve at room temperature, and concentrate under reduced pressure at 35°C to obtain sorafenib 2-aminopyrimidine cocrystal A.
Embodiment 2
[0036] Take 20 mg of sorafenib free base and 4.09 mg of 2-aminopyrimidine, add 0.8 mL of tetrahydrofuran to dissolve it, and volatilize it in the open at 40 ° C to obtain sorafenib 2-aminopyrimidine cocrystal A.
Embodiment 3
[0038] Add 20 mg of sorafenib free base and 4.09 mg of 2-aminopyrimidine into a mortar, add 0.8 mL of tetrahydrofuran, and grind to obtain sorafenib 2-aminopyrimidine cocrystal A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com