A kind of preparation method and application of ultraviolet curing type silicone oil containing methacrylate
A technology containing methacrylate and methacrylate, applied in special paper, papermaking, coating and other directions, can solve the problems of easy crosslinking, low acrylate grafting efficiency, complicated and expensive preparation of initiators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] Example 1
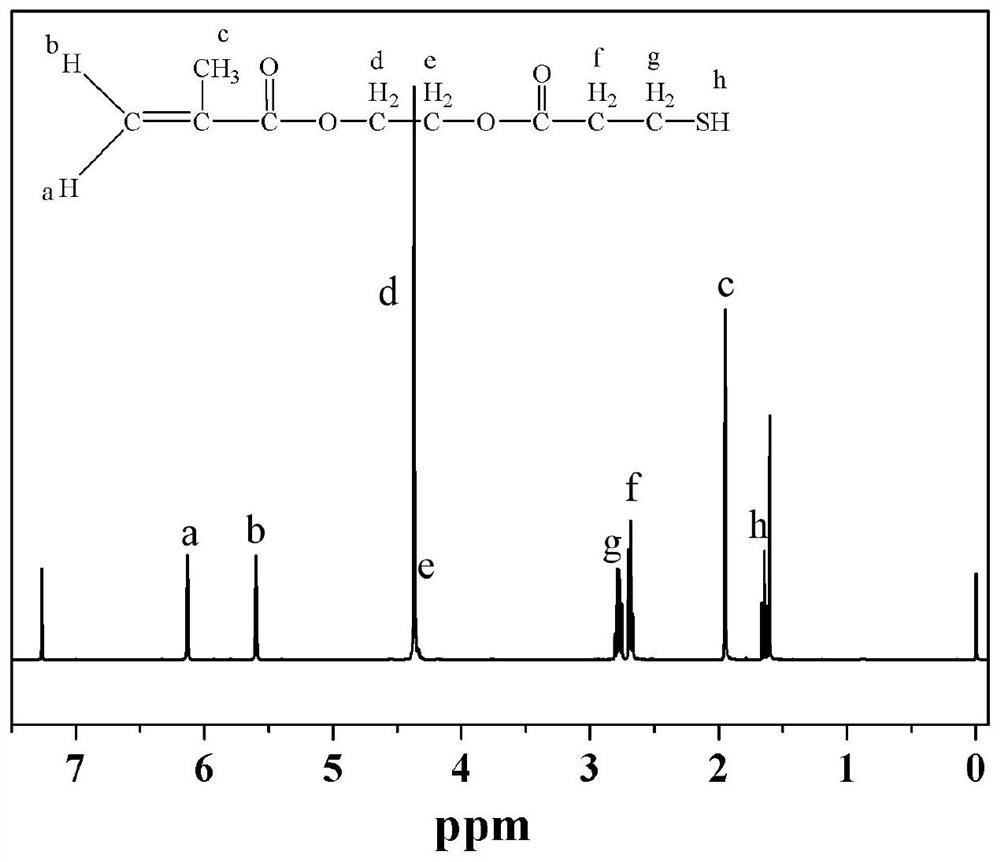
[0033] Mercapto group-containing methacrylate compound, methacrylic acid-3-mercapto-propionyloxy ethyl, hydroxyethyl acrylate mercaptopropionic acid obtained by esterification with methacrylic acid, the reaction of equation [3]:
[0034]
[0035] [3] Synthesis of methacrylic acid, 3-mercapto-propionyloxy-ethyl ester
[0036] Methacrylate, 2-hydroxyethyl methacrylate (13.00 g; 0.10 mol), 3- mercaptopropionic acid (11.13 g; 0.105 mol), p-toluenesulfonic acid (1.00 g) as an esterification reaction catalyst, hydroquinone (0.12 g) as a polymerization inhibitor, benzene (15.00 mL) as a water, was gradually added to a 250 mL three-neck flask. After loading three bottles trap placed in an oil bath of slowly heated to reflux, the reaction temperature 8 h. After completion of the reaction product was cooled to, washed with a dilute solution of 3% sodium bicarbonate 3 times, washed with distilled water until neutral, dried over anhydrous sodium sulfate overnight. The sol...
Example Embodiment
[0037] Example 2
[0038] Synthesis of polymethyl methacrylate silicone material of the end structure (PSi-MMA) is.
[0039] Epoxy-terminated methoxy poly siloxane structural formula [2], made, Figure 4 Epoxy-terminated methoxy poly siloxane NMR spectrum, the structure of the one-NMR proton peak, by integration calculation, n = 14, molecular weight of 1300.
[0040] To 250 mL three-necked flask with a constant pressure funnel, and a bottom magnetic stir bar were added to epoxy-terminated methoxy poly siloxane (13.00 g, 0.01 mol), triethylamine (0.08 g, 0.0008 mol) and solvent of acetone (50 mL). Stirring at room temperature, methyl acrylate, 3-mercapto-propionyloxy-ethyl ester (4.36 g, 0.02 mol) in acetone (10 mL) was added dropwise to the reaction solution, and epoxy-terminated silicone with methoxy polyethylene the molar ratio of acrylate, 3-methyl-mercapto-propionyloxy-ethyl ester 1: 2, epoxy-terminated methoxy poly siloxane was added dropwise 10 min, the reaction was continued...
Example Embodiment
[0041] Example 3
[0042] Synthesis of polymethyl methacrylate silicone material of the end structure (PSi-MMA) is.
[0043] Example 2 except that the epoxy-terminated methoxy poly siloxane has a molecular weight 2500, n = 30.
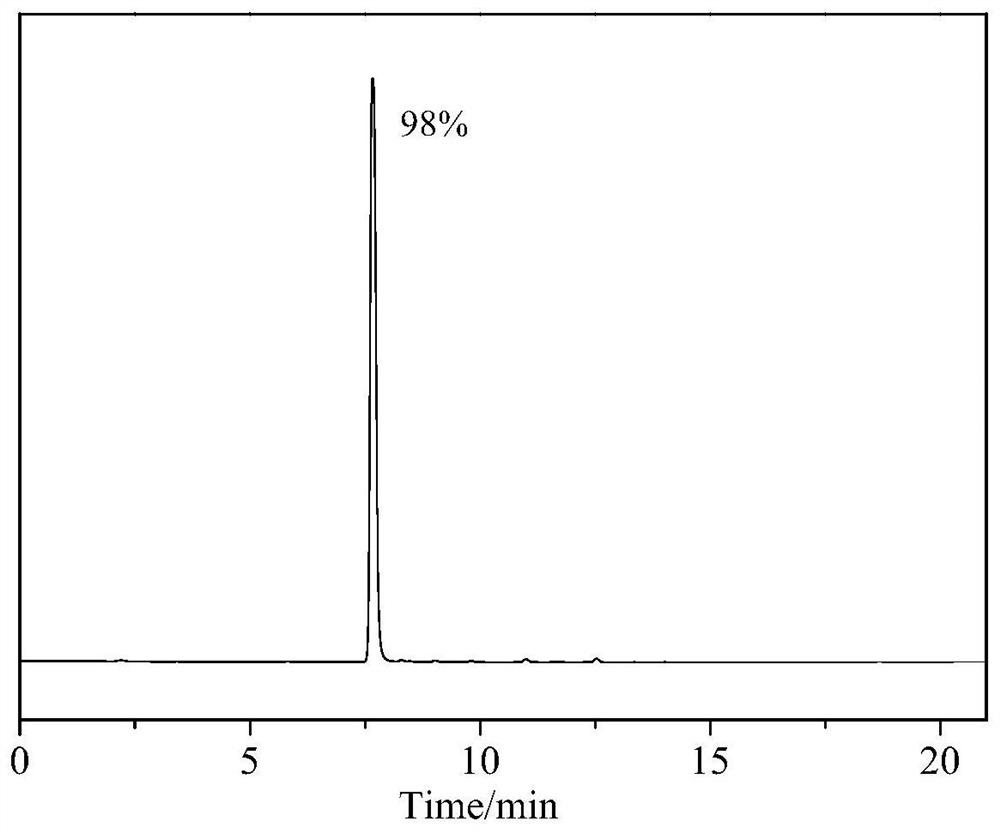
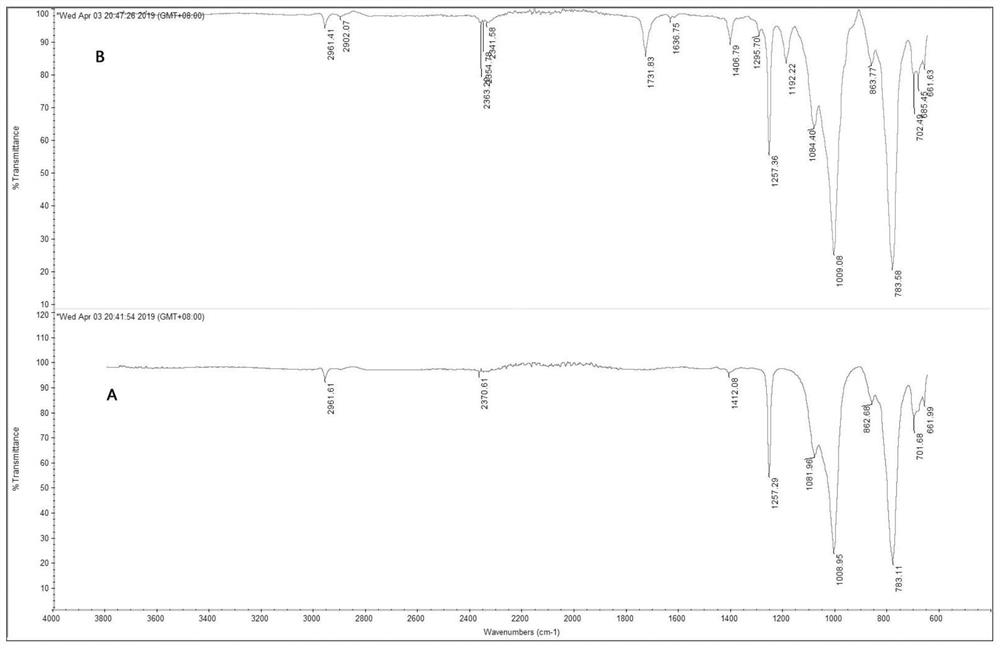
[0044] In other embodiments without changing the conditions of Example 2, the preparation process epoxy-terminated siloxane and poly methoxy-yl methacrylate, 3-mercapto-propionyloxy-ethyl weights were: 25.0 g and 4.36 g , the same amount of solvent can be used tetrahydrofuran, ethyl acetate, toluene, xylene, acetone, methyl ethyl ketone and the like in place of, the same operation, characterized by IR and NMR spectra confirm the successful preparation of a PSi-MMA, molecular weight 2900, methacrylic acid ester grafted 97%. Reference numeral PSi-MMA02.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Release force | aaaaa | aaaaa |
| Contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com