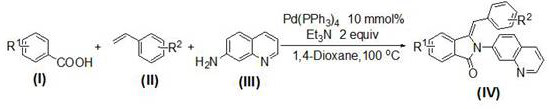
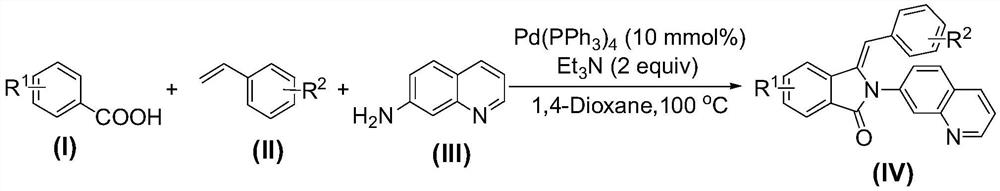
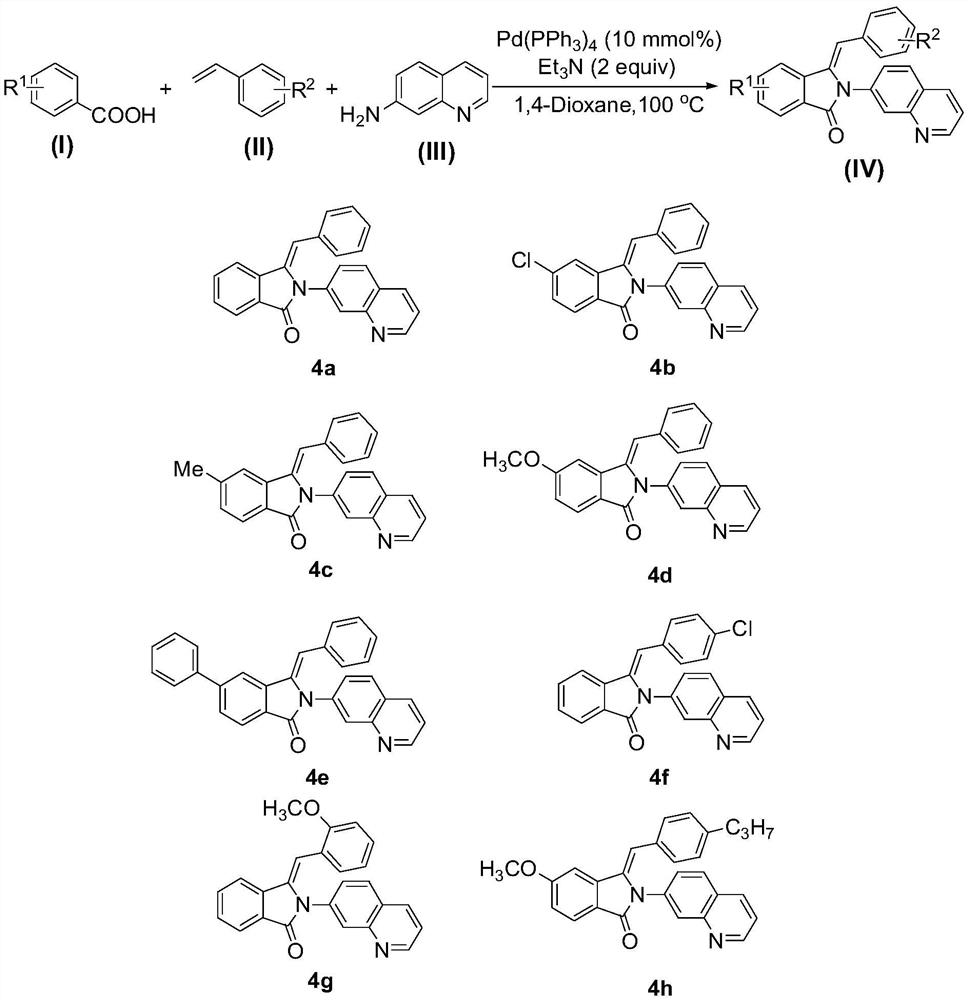
Method for preparing 3-benzylidene-2-(7'-quinoline)-2,3-dihydro-isoindol-1-one compounds
A ketone compound and benzylidene technology is applied in the field of preparation of pharmaceutical synthesis intermediates, and can solve the problems of low reaction efficiency and many reaction steps, and achieve the effects of convenient post-processing, low equipment requirements and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of product 4a
[0024]
[0025] At room temperature, add 610mg (5mmol) of benzoic acid 1a, 625mg (6mmol) of styrene 2a and 721mg (5mmol) of 7-aminoquinoline 3a into a 25mL round-bottomed flask, and then add 578mg (0.5mmol) of tetrathree Phenylphosphine palladium, 15 mL of 1,4-dioxane and 1010 mg (10 mmol) of triethylamine were stirred at 100° C. for 8 hours. After the reaction is complete, add 15 mL saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and perform 200-300 mesh silica gel column chromatography The pure product of 4a was obtained (1411 mg, 81% yield, pale yellow solid).
[0026] 1 H NMR (400MHz, CDCl 3 )δ8.86(dd, J=4.2,1.7Hz,1H),7.98(dd,J=11.2,4.6Hz,2H),7.89(d,J=7.8Hz,1H),7.69(td,J=7.6 ,1.1Hz, 1H),7.60–7.54(m,2H),7.48(dd,J=7.3,1.4Hz,1H),7.33–7.27(m, 2H),6.81(s,1H),6.67(dd, J=11...
Embodiment 2
[0027] Embodiment 2: the preparation of product 4b
[0028]
[0029] At room temperature, add 783mg (5mmol) of p-chlorobenzoic acid 1b, 625mg (6mmol) of styrene 2a and 721mg (5mmol) of 7-aminoquinoline 3a into a 25mL round bottom flask, and then add 578mg (0.5mmol) Tetrakistriphenylphosphinepalladium, 15mL of 1,4-dioxane and 1010mg (10mmol) of triethylamine were stirred at 100°C for 8 hours. After the reaction is complete, add 15 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and perform 200-300 mesh silica gel column chromatography The pure product of 4b was obtained (1589 mg, 83% yield, pale yellow solid).
[0030] 1 H NMR (400MHz, CDCl 3 )δ8.84(dd, J=4.2,1.7Hz,1H),7.97(dd,J=8.3,1.7Hz,1H),7.92(d,J=8.1Hz,1H),7.87(d,J=1.6 Hz,1H),7.59 (dd,J=8.2,1.4Hz,1H),7.53(dd,J=8.1,1.7Hz,1H),7.48(dd,J=7.4,1.4Hz,1H),7.32–7.28 (m,2H...
Embodiment 3
[0032] Embodiment 3: the preparation of product 4c
[0033]
[0034] At room temperature, 681mg (5mmol) of p-toluic acid 1c, 625mg (6mmol) of styrene 2a and 721mg (5mmol) of 7-aminoquinoline 3a were added to a 25mL round bottom flask, and then 578mg (0.5mmol) of ) tetrakistriphenylphosphinepalladium, 15mL of 1,4-dioxane and 1010mg (10mmol) of triethylamine, stirred at 100°C for 8 hours. After the reaction is complete, add 15 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and perform 200-300 mesh silica gel column chromatography The pure product of 4c was obtained (1667 mg, 92% yield, pale yellow solid).
[0035] 1 H NMR (400MHz, CDCl 3 )δ8.84(dd, J=4.2,1.6Hz,1H),7.96(dd,J=8.3,1.6Hz,1H),7.87(d,J=7.8Hz,1H),7.68(s,1H), 7.57(d, J=8.2Hz, 1H), 7.47(dd, J=7.3, 1.2Hz, 1H), 7.37(d, J=7.2Hz, 1H), 7.31– 7.27(m, 2H), 6.77(s ,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com