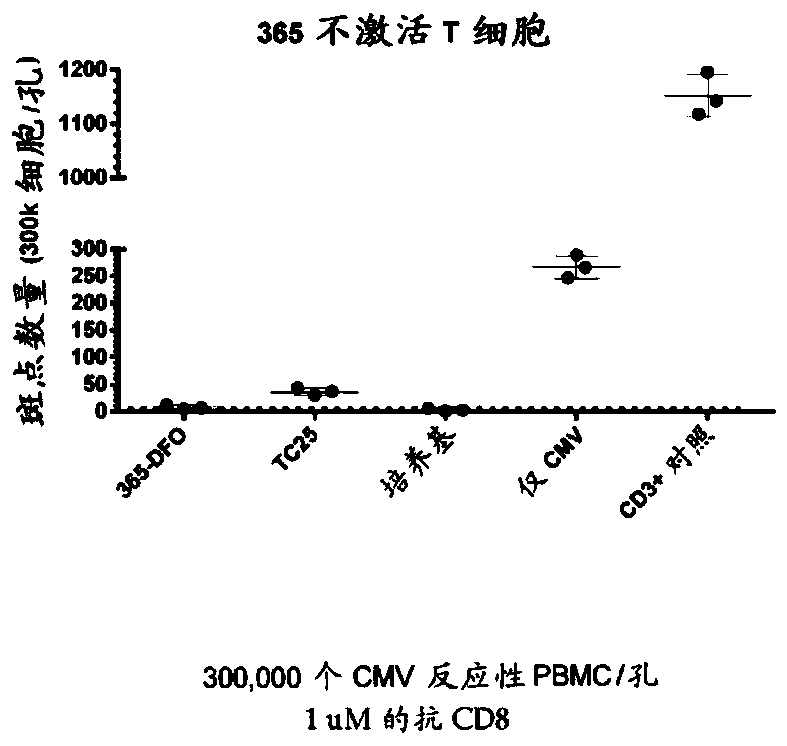
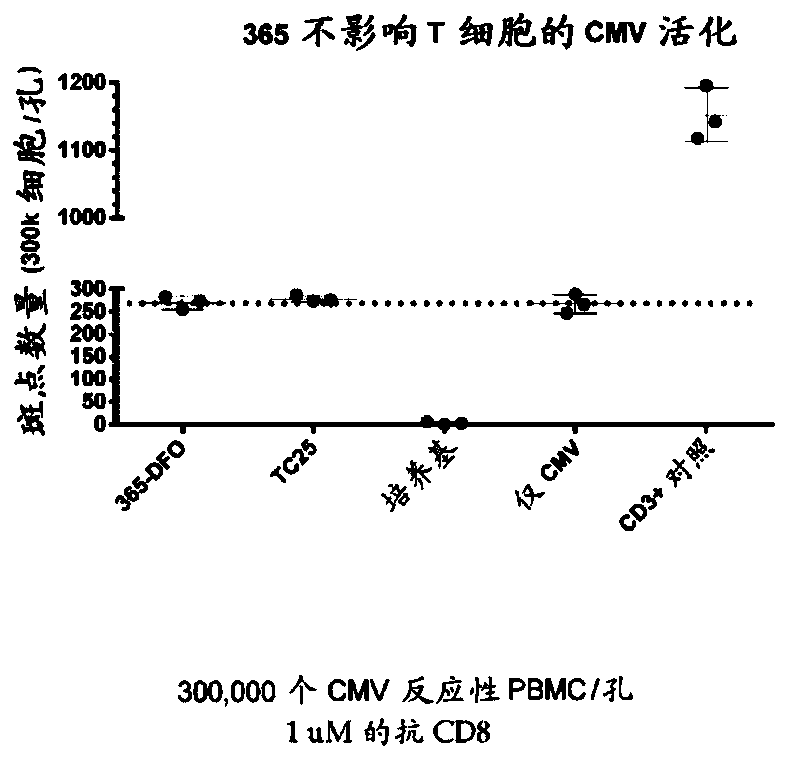
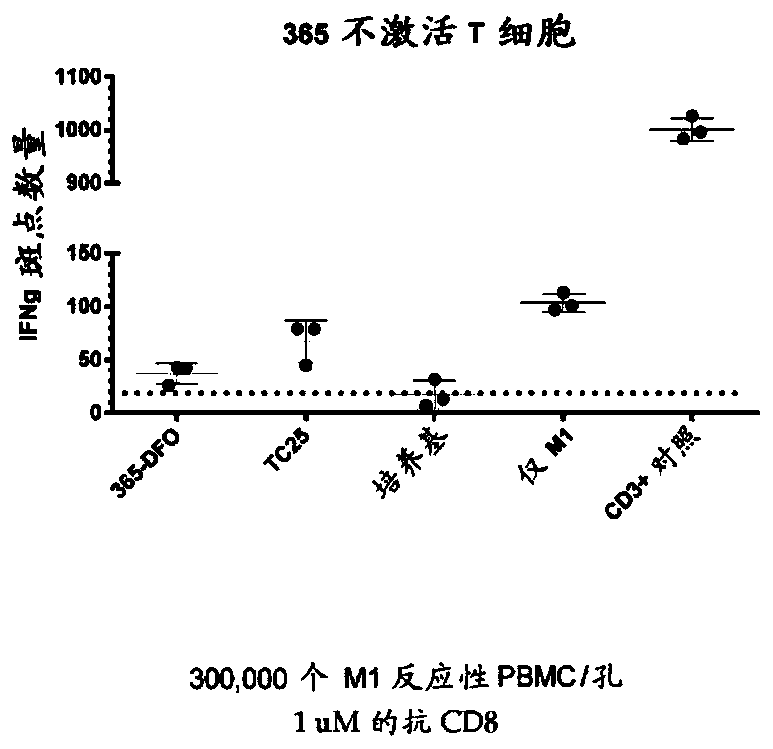
Cd8a-binding fibronectin type iii domains
A technology of structural domains and proteins, applied in the direction of recombinant DNA technology, animal/human proteins, hybrid peptides, etc., can solve the problems of changes in the number and location of immune cells, and the inability to reflect dynamic and spatial information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0119] The present invention also provides the following non-limiting embodiments.
[0120] 1) An isolated FN3 domain that specifically binds to the human CD8A protein comprising the amino acid sequence of SEQ ID NO:35.
[0121] 2) The isolated FN3 domain of embodiment 1, wherein the FN3 domain cross-reacts with the cynomolgus monkey CD8A protein comprising the amino acid sequence of SEQ ID NO:271.
[0122] 3) The isolated FN3 domain according to embodiment 2, wherein
[0123] a) The FN3 domain is based on the Tencon sequence of SEQ ID NO:1;
[0124] b) The FN3 domain is based on the Tencon27 sequence of SEQ ID NO: 4; and / or
[0125] c) The FN3 domain is isolated from a library containing the sequence of SEQ ID NO: 2, 3, 5, 6, 7 or 8.
[0126] 4) The isolated FN3 domain of embodiment 3, wherein the FN3 domain is conjugated to a second molecule.
[0127] 5) The isolated FN3 domain of embodiment 4, wherein the second molecule is a detectable label.
[0128] 6) The isolated FN3 domain of embod...
Embodiment 1
[0149] Example 1. Construction of a TENCON library with randomized loops
[0150] Tencon (SEQ ID NO:1) is an immunoglobulin-like scaffold, fibronectin type III (FN3) domain, which is designed from the consensus sequence of 15 FN3 domains of human tenascin-C (Jacobs et al. , Protein Engineering, Design, and Selection, 25:107-117,2012; U.S. Patent No. 8,278,419). The crystal structure of Tencon shows six surface exposed loops that connect seven β-strands. These loops or selected residues within each loop can be randomized to construct a library of fibronectin type III (FN3) domains that can be used to select novel molecules that bind to specific targets.
[0151] Tencon:
[0152] LPAPKNLVVSEVTEDSLRLSWTAPDAAFDSFLIQYQESEKVG EAINLTVPGSERSYDLTGLKPGTEYTVSIYGVKGGHRSNPLSAEFT T (SEQ ID NO 1):
[0153] Use tencon scaffolds and various design strategies to generate various libraries. In general, libraries TCL1 and TCL2 produced good conjugates. The production of TCL1 and TCL2 libraries is des...
Embodiment 2
[0206] Example 2: Generation of TENCON library with alternative binding surface
[0207] The choice of residues to be randomized in a particular library design determines the overall shape of the interaction surface generated. X-ray crystallographic analysis of the maltose binding protein (MBP)-binding FN3-domain-containing scaffold protein selected from the library in which the BC, DE and FG loops were randomized showed that it has a largely curved interface suitable for the active site of MBP (Koide et al., Proc Natl Acad Sci USA 104:6632-6637, 2007). In contrast, it was found that the ankyrin repeat scaffold protein selected to bind to MBP has a flatter interaction surface and binds to the outer surface of MBP away from the active site (Binz et al., Nat Biotechnol 22:575-582, 2004 ). These results indicate that the shape of the binding surface of the scaffold molecule (curved versus flat) can determine which target proteins or specific epitopes on those target proteins can b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com