Methods and compositions for adaptive immune modulation
A composition and allergen technology, applied in the direction of drug combination, pharmaceutical formula, allergic diseases, etc., can solve problems such as deficiencies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
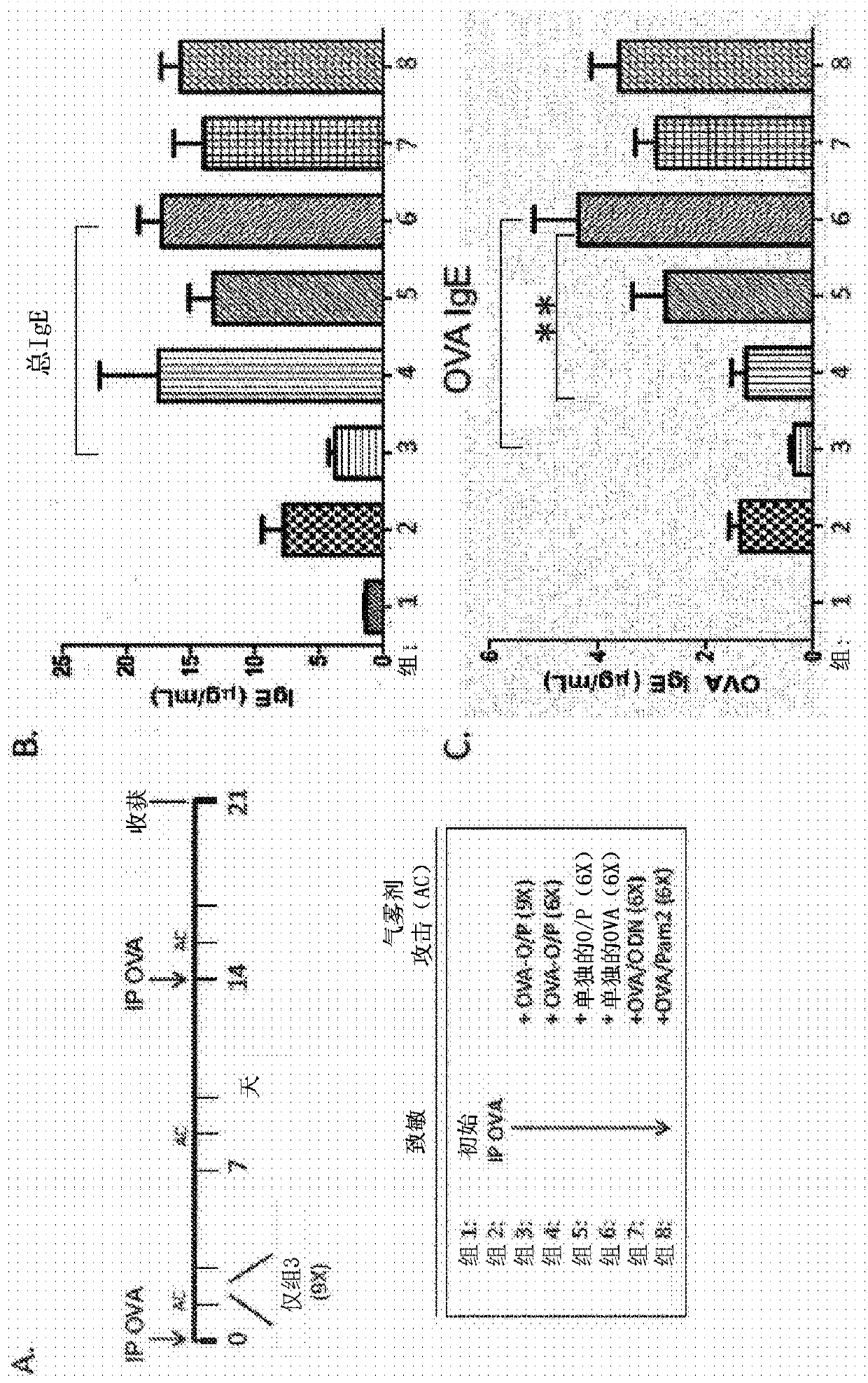
[0191] Example 1 Activation of lung innate immunity to regulate immune response in asthma
[0192] In the present study, the inventors investigated the possible role of inducible innate resistance in the regulation of the immune response in asthma using an experimental mouse model of asthma. Analysis of serum immunoglobulins and differential cell counts of bronchoalveolar lavage (BAL) fluid revealed that aerosol administration of O / P with OVA significantly reduced OVA-specific IgE levels as well as eosinophils in BAL. In contrast, the inventors observed an increase in serum IgG2a levels, a marker of Th1 immunity. Taken together, our findings suggest that activation of lung innate immunity with O / P in combination with specific antigens can switch allergic to non-allergic immune responses.
[0193] A. Results
[0194] Nebulized OVA-O / P decreased total IgE serum levels and OVA-IgE serum levels. Preliminary studies with aerosolized O / P have demonstrated a robust defense respons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com