Flavonoid derivative with tumor cell inhibition and preparation method and application thereof
A technology of tumor cells and derivatives, applied in antineoplastic drugs, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as side effects, intolerance and drug resistance, and failure to reduce postoperative recurrence and metastasis , to achieve good anti-proliferation activity and high anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
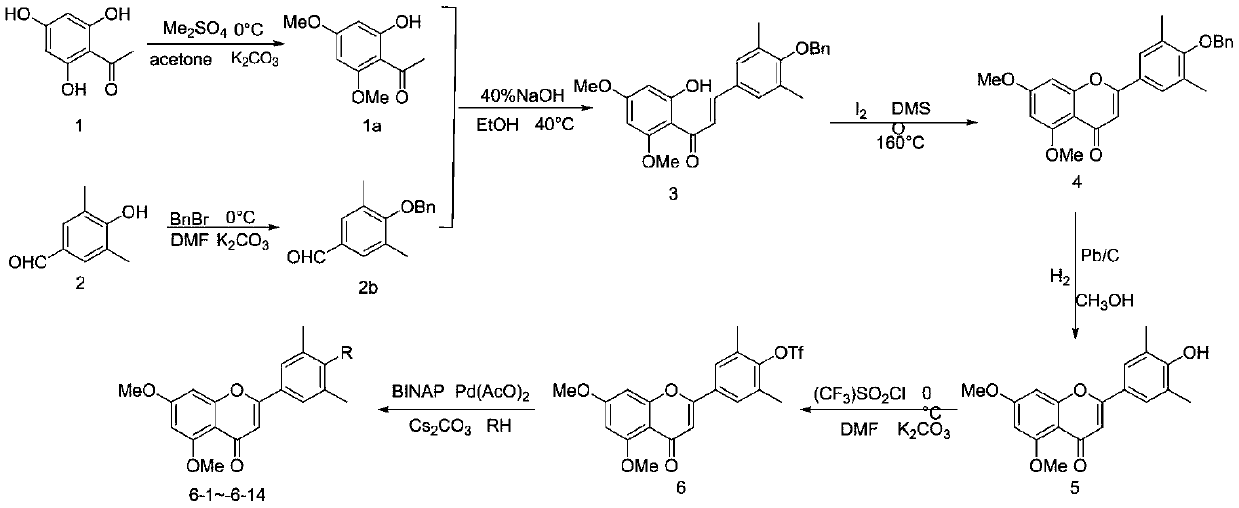
[0017] (1) Synthesis of compound 1a: at room temperature, 2,4,6-trihydroxyacetophenone 1 (1.0 equivalents) and K 2 CO 3 (2.0 equivalents) was dissolved in acetone and dropped into Me 2 SO 4 (2.0eq), the temperature was raised to 40° C., and the reaction was monitored by TLC for about 4 hours. The reaction was filtered and washed 3 times with acetone. Evaporation of the organic phase afforded 1a in 98% yield.
[0018] (2) Synthesis of compound 2b: 3,5-dimethyl p-hydroxybenzaldehyde (6mmol, 1.0eq), K 2 CO 3 (12mmol, 2.0eq) was dissolved in anhydrous DMF (40mL), cooled to 0 degrees. Then BrBn (6.6mmol, 1.1eq) was added dropwise, and after the drop, the temperature was slowly raised to room temperature. The reaction was monitored by TLC. After the reaction was completed, 50 mL of water was added, extracted with EA 20 mL×3, the organic layers were combined, washed three times with 50 mL of water, washed three times with 20 mL of saturated saline, and washed three times with a...
Embodiment 2
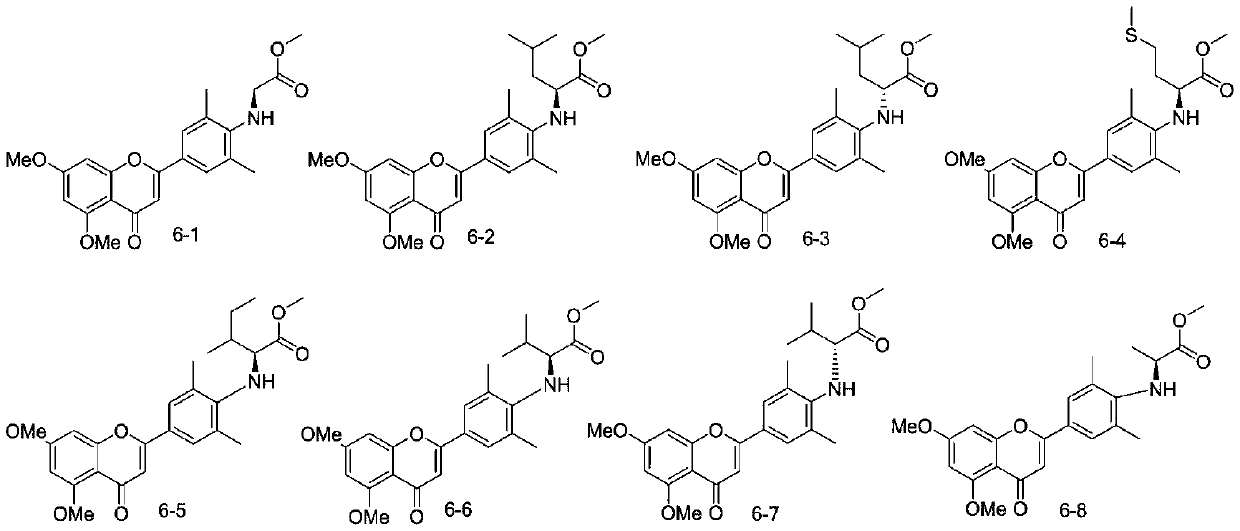
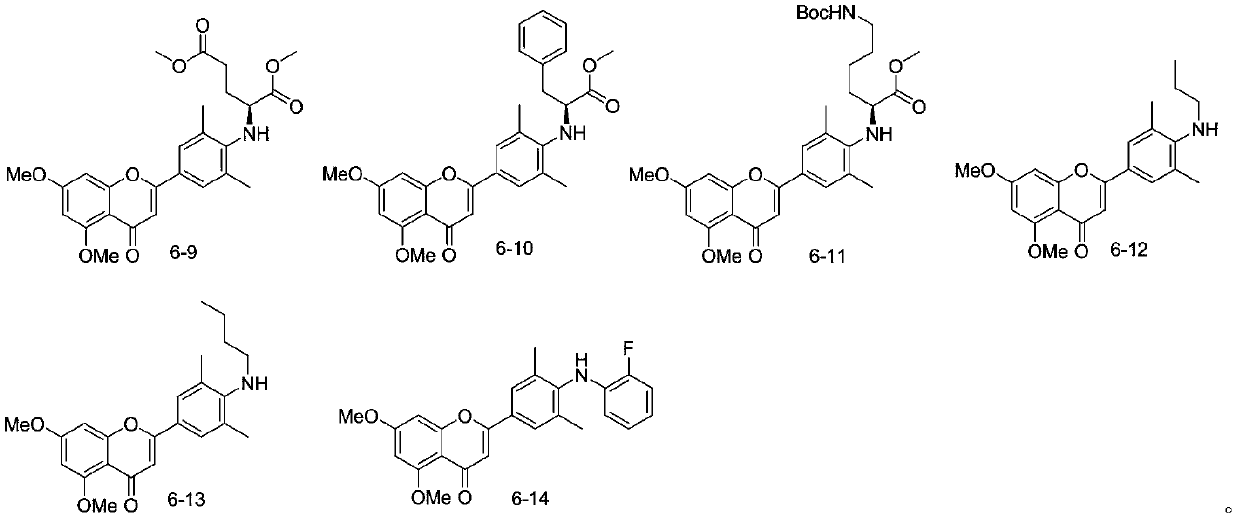
[0025] In order to further confirm the accuracy of the compound structure, nuclear magnetic resonance was used to detect the target product 6-1-6-14 compound to obtain the following results:
[0026] Compound 6-1: 60% yield, m.p.: 134.3-135.7℃, 1 HNMR (400MHz, CDCl 3 )δ: (ppm) 7.49 (s, 2H, Ar-H), 6.56 (s, 2H, Ar-H, C=C-H), 6.35 (s, 1H, Ar-H), 4.26 (s, 1H, NH ),3.94(s,5H,OCH 3, COCH2),3.91(s,3H,OCH 3 ),3.77(s,3H,OCH 3 ),2.38(s,6H,ArCH 3 ); 13 C NMR (151MHz, CDCl 3 )δ: (ppm) 177.7, 172.4, 163.8, 161.1, 160.8, 159.9, 148.9, 127.6, 126.8, 123.7, 109.2, 107.4, 96.0, 92.8, 56.4, 55.7, 52.4, 49.3, 19.1. C 22 h 24 o 6 N,[M+H] + m / z: 398.1598, found: 398.1596.
[0027] Compound 6-2: 55% yield, m.p.: 192.7-193.3°C; 1 HNMR (400MHz, CDCl 3 )δ: (ppm)7.47((s,2H,Ar-H),6.55-6.56(m,1H,Ar-H,C=C-H),6.34(d,J=2.0Hz,1H,Ar-H) ,6.36(d,J=2.0Hz,1H,Ar-H),4.10-4.15(m,1H,NH CH ),3.93(s,3H,OCH 3 ),3.90(s,3H,OCH 3 ),3.61(s,3H,OCH 3 ),2.36(s,6H,ArCH 3 ),1.61-1.80(m,3H,CH 2 CH), 0.97(t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com