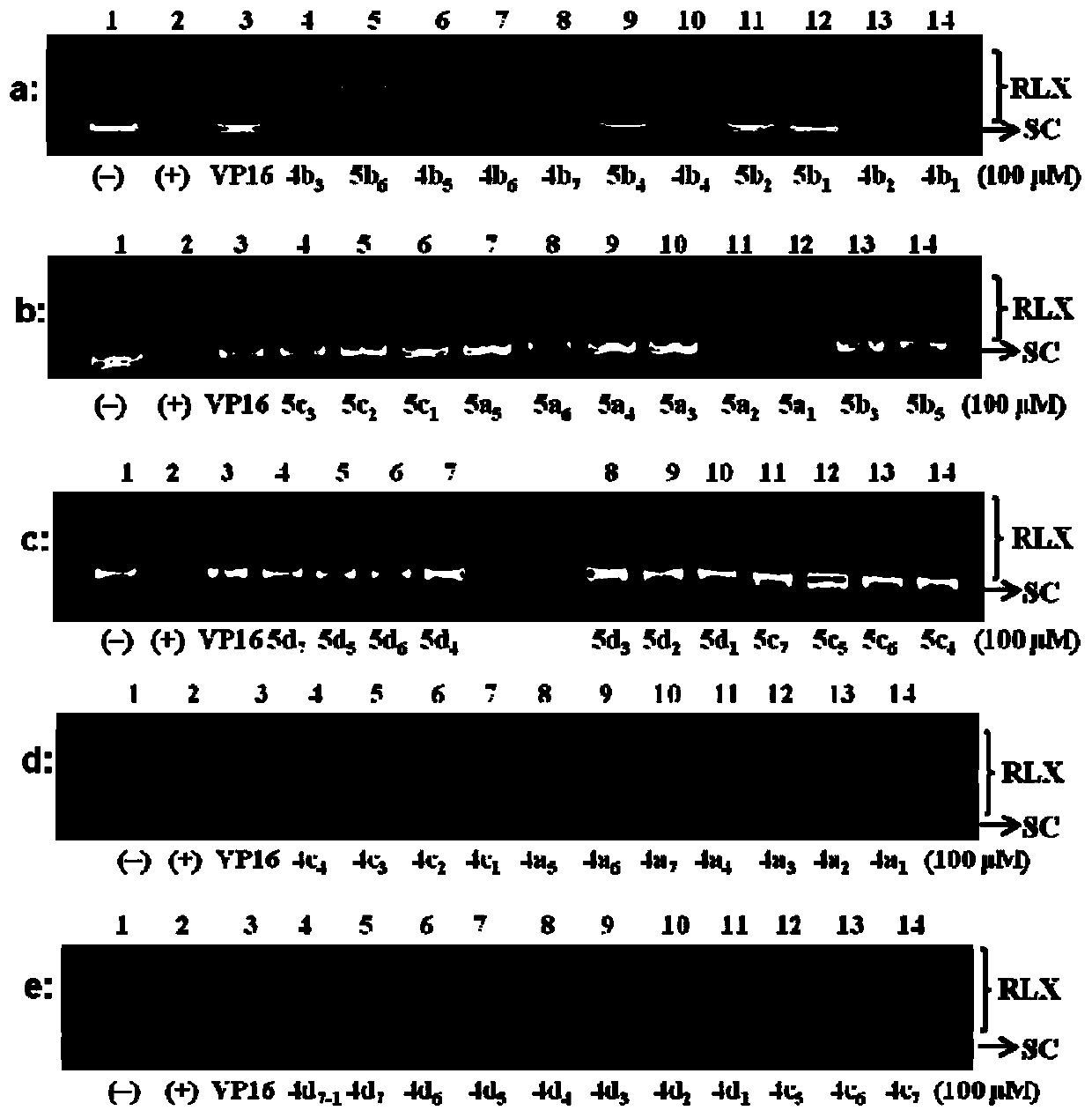
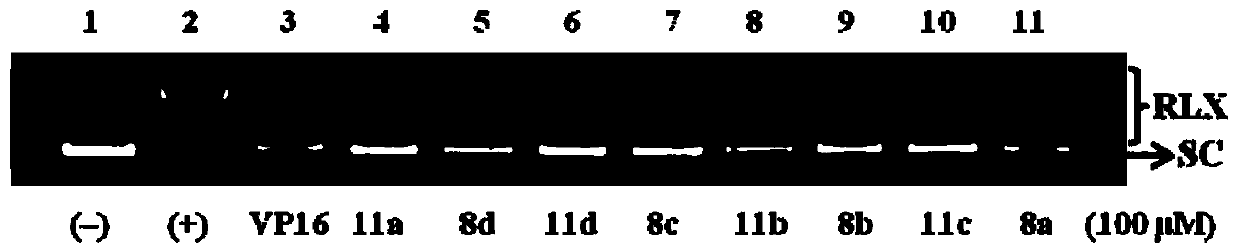
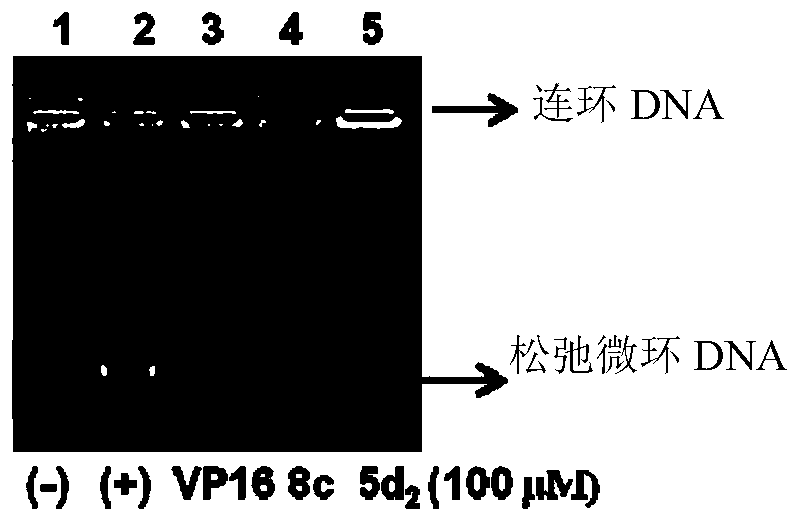
Anthracedione derivatives and their preparation methods and applications
A derivative, the technology of anthracenedione, which is applied in the field of anthracenedione derivatives and its preparation, can solve the problems of high therapeutic index, high toxicity, and few types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1 .(S)-2-(4-(((4-(hexyloxy)-9,10-dioxo-9,10-dihydroxyanthracene-1-yl)oxy)methyl)benzamide)propane acid (compound 5a 1 ) preparation
[0182] 1. Preparation of 1-(hexyloxy)-4-hydroxyanthracene-9,10-dione (intermediate 1a)
[0183] Under ice-bath conditions, weigh 1,4-dihydroxyanthracenedione (4.80g, 0.02mol) and dissolve it in redistilled DMF (35mL), add NaH (0.48g, 0.02mol) in batches, and the resulting solution was The reaction was carried out under nitrogen protection for 30 minutes, and then the temperature was raised to 60-75°C to continue the reaction for 1 hour, 1-bromo-n-hexane (14.0 mL, 0.10 mol) was added, and the resulting solution was reacted at 95°C for 42 hours until the reaction was completed by TLC monitoring. The reaction solution was poured into ice water, extracted twice with dichloromethane (2×15mL), the organic phase was washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, and the solvent was concentrat...
Embodiment 2
[0193] Example 2. (S)-2-(4-(((4-(hexyloxy)-9,10-dioxo-9,10-dihydroxyanthracene-1-yl)oxy)methyl)benzamide)-3 -Phenylpropionic acid (compound 5a 2 ) preparation
[0194] According to compound 5a 1 The preparation method prepares compound 5a 2 . Yellow solid, 92.9% yield; melting point 217–219°C. 1 H NMR (600MHz, DMSO–d 6 )δ:12.86(brs,1H),8.65(d,J=7.1Hz,1H),8.09–8.02(m,2H),7.86–7.82(m,4H),7.69(d,J=8.2Hz,2H ),7.60(d,J=9.5Hz,1H),7.56(d,J=9.5Hz,1H),7.32(d,J=7.3Hz,2H),7.27(t,J=7.6Hz,2H), 7.18(t, J=7.3Hz, 1H), 5.33(s, 2H), 4.64–4.57(m, 1H), 4.09(t, J=6.4Hz, 2H), 3.21(dd, J 1 =13.7,J 2 =4.1Hz,1H),3.08(dd,J 1 =13.6,J 2 =10.6Hz,1H),1.77(q,J=7.0Hz,2H),1.52(q,J=7.4Hz,2H),1.39–1.28(m,4H),0.90(t,J=7.0Hz,3H ); ESI–MS m / z:604.5[M–H] – .
Embodiment 3
[0195] Example 3. (S)-2-(4-(((4-(hexyloxy)-9,10-dioxo-9,10-dihydroxyanthracene-1-yl)oxy)methyl)benzamide)-3 -Methylbutanoic acid (compound 5a 3 ) preparation
[0196] According to compound 5a 1 The preparation method prepares compound 5a 3 . Yellow solid, yield 84.5%, melting point 202–205°C; 1 H NMR (600MHz, DMSO–d 6 )δ:12.62(brs,1H),8.42(d,J=8.2Hz,1H),8.10–8.03(m,2H),7.95(d,J=8.3Hz,2H),7.85–7.81(m,2H ),7.72(d,J=8.3Hz,2H),7.62(d,J=9.5Hz,1H),7.57(d,J=9.5Hz,1H),5.35(s,2H),4.30(dd,J 1 =7.9,J 2 =7.1Hz, 1H), 4.09(t, J=6.4Hz, 2H), 2.24–2.16(m, 1H), 1.77(q, J=7.0Hz, 2H), 1.52(q, J=7.4Hz, 2H ),1.38–1.29(m,4H),1.04–0.93(m,6H),0.90(t,J=7.0Hz,3H); ESI–MS m / z:556.2[M–H] – .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com