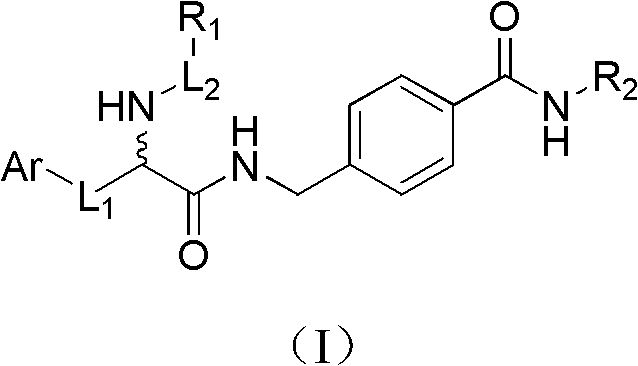
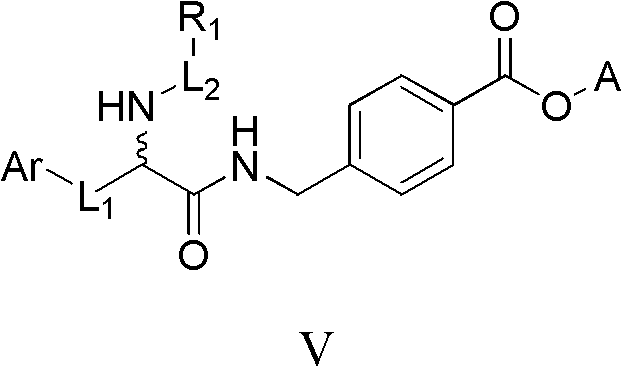
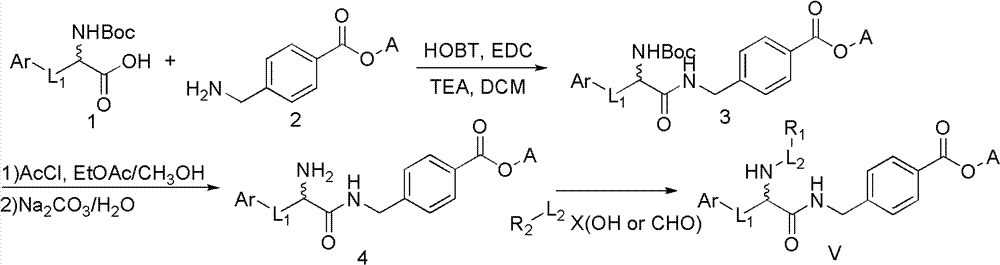
Histone deacetylase inhibitor containing alpha amino acid structure and application thereof
A technology of alkylamino and hydroxyl group, which is applied in the field of pharmaceutical preparations and achieves the effect of good development prospect and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] Preparation of methyl 4-((2-(tert-butoxycarbonylamino)-3-(1H-indol-3-yl)propionamido)methyl)benzoate
[0056] Dissolve N-tert-butoxycarbonyl tryptophan (Boc-Trp-OH, 6.08g, 20.0mmol) in anhydrous dichloromethane (DCM, 100mL), and cool in an ice-salt bath until the temperature of the reaction solution drops to -15°C , add 1-hydroxybenzotriazole (HOBT, 3.24g, 24.0mmol) to the above solution, continue stirring for 15min, then add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCHCl, 7.66 g, 40 mmol) and triethylamine (TEA, 12.5 mL, 90 mmol). Stirring was continued for 30 min before methyl p-aminomethylbenzoate (4.03 g, 20.0 mmol) was added. The mixture was quenched after stirring at room temperature for 8 h. The reaction solution was washed 3 times with 80 mL water, anhydrous Na 2 SO 4 dry. The solvent was evaporated to dryness, and ethyl acetate was recrystallized to obtain a white solid (7.3g, 81.8%); M.p.178-180°C; 1 H NMR (DMSO-d 6 )δ: 1...
Embodiment 2
[0058]
[0059] Preparation of methyl 4-((2amino-3-(1H-indol-3-yl)propionamide)methyl)benzoate
[0060] To 4-((2-(tert-butoxycarbonylamino)-3-(1H-indol-3-yl)propionamido)methyl)methyl benzoate (Example 1, 11.40g, 0.025mol ) into a mixture solution of methanol (25 mL) and ethyl acetate (25 mL) was slowly added dropwise with acetyl chloride (8 mL), and after the dropwise addition was completed, the temperature was raised to reflux for 2 h. Then the solvent and excess HCl were spin-dried under reduced pressure and then added 5% Na 2 CO 3 Aqueous solution (80 mL), followed by addition of ethyl acetate for extraction, washing, drying, and concentration gave a white solid (8.9 g, 91.8%). 1 HNMR (DMSO-d 6 )δ11.08(s, 1H, NH Indole ), 9.12(t, 1H, NH), 8.34(bs, 3H, NH+NH 2 ), 7.84 (d, 2H, J=8.4, ArH), 7.67 (d, 1H, J=7.8, ArH), 7.39 (d, 1H, J=8.1, ArH), 7.20 (d, 3H, ArH), 7.10(t, 1H, ArH), 7.01(t, 1H, ArH), 4.44-4.27(m, 2H, CH 2 NH), 4.05 (bs, 1H, CHα Trp), 3.40-3.20 (m, 2H, CH...
Embodiment 3
[0062]
[0063] A general procedure for introducing amide side chains using various acid chloride reagents: methyl 4-((2amino-3-(1H-indol-3-yl)propionamide)methyl)benzoate (Example 2, 1.0 eq ) and triethylamine (TEA, 2.0eq) in dichloromethane were added dropwise acid chloride (1.2eq) or sulfonyl chloride (1.2eq). The solution was stirred at room temperature until TLC showed the reaction was complete. The reaction solution was concentrated, added with water, extracted with ethyl acetate, and washed with brine. The organic layer was dried and concentrated under reduced pressure, and the obtained crude product was separated and purified by column chromatography.
[0064] A general procedure for introducing amide side chains using various carboxylic acid reagents: Add HOBT (1.5 eq) to various carboxylic acid reagents (1.2 eq) in dichloromethane, stir for 15 min in ice bath and then add EDCI (1.2 eq) and TEA (4.5 eq). Stirring was continued for 20 min before methyl 4-((2amino-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com