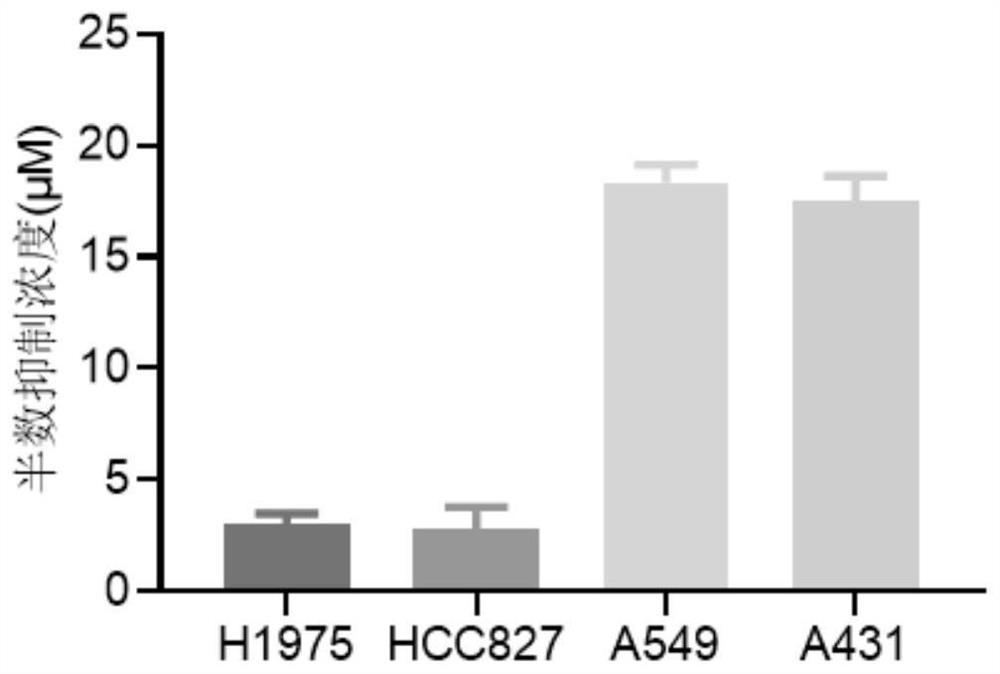
2, 4-disubstituted quinazoline compound for inhibiting EGFR (epidermal growth factor receptor) as well as preparation method and application of 2, 4-disubstituted quinazoline compound
A technology of quinazolines and compounds, which is applied in the field of antitumor pharmacy, can solve the problems of inability to activate downstream substrates, and achieve good antiproliferative activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
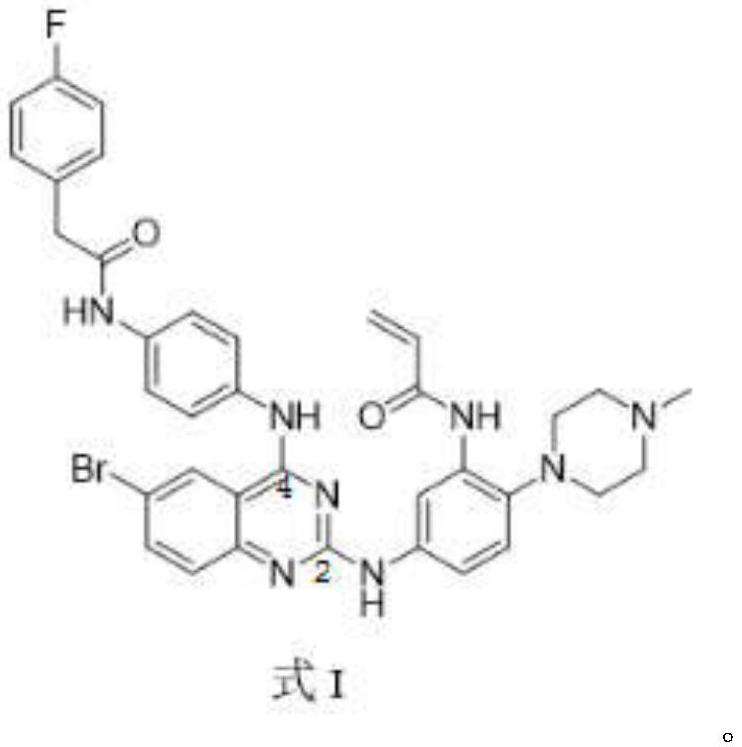
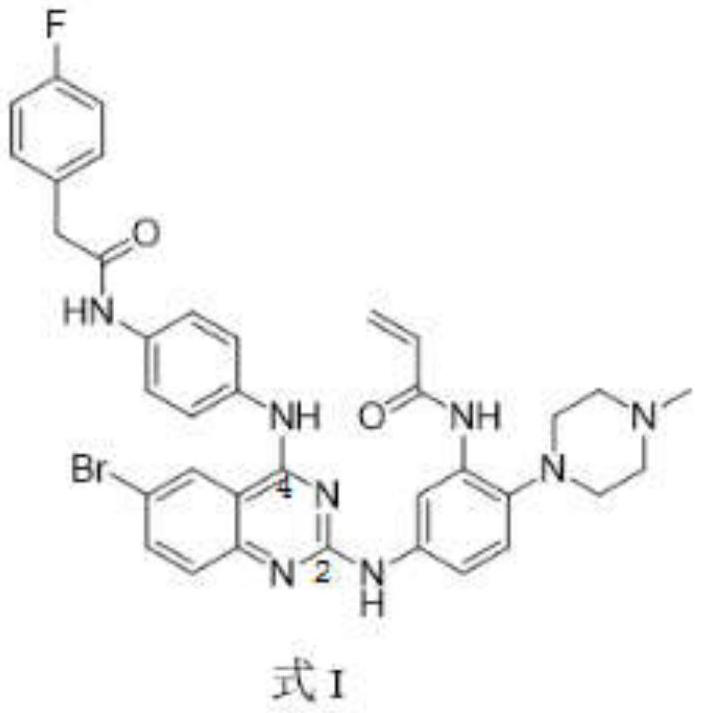
[0032] A 2,4-disubstituted quinazoline compound for inhibiting EGFR, characterized in that: the general formula of the compound structure shown below or a pharmaceutically acceptable salt thereof is provided:
[0033]
[0034] Wherein, F represents a fluorine atom, the substituent on the 4th position is a fluorophenylacetamide group, the substituent on the 2nd position is a phenyl group, and the substituents on the phenyl group are acrylamide and piperazine.
Embodiment 2
[0036] A preparation method of 2,4-disubstituted quinazoline compounds for inhibiting EGFR, the steps are as follows:
[0037] (1) Intermediate 1 is synthesized:
[0038] Dissolve 2,4-dichloroquinazoline in isopropanol and stir, add p-phenylenediamine and DIPEA in turn, stir evenly, heat the reaction solution to 75°C for 5h, and filter under reduced pressure to obtain a yellow solid. After separation by column chromatography, intermediate 1 is obtained; wherein, the mass dosage is 2,4-dichloroquinazoline: isopropanol: p-phenylenediamine: DIPEA=260:8:80:0.6;
[0039] (2) the intermediate 1 was dissolved in tetrahydrofuran and stirred in an ice bath, p-fluorophenylacetyl chloride was added, triethylamine was added dropwise, then the temperature was raised to 20° C. for 3 hours, and concentrated under reduced pressure. After the crude product was separated by column chromatography Intermediate 2 was obtained; wherein, intermediate 1: tetrahydrofuran: p-fluorophenylacetyl chlorid...
Embodiment 3
[0048] A preparation method of 2,4-disubstituted quinazoline compounds for inhibiting EGFR, the steps are as follows:
[0049] (1) Intermediate 1 is synthesized:
[0050] Dissolve 2,4-dichloroquinazoline in isopropanol and stir, add p-phenylenediamine and DIPEA in turn, stir evenly, heat the reaction solution to 85°C for 3 hours, and filter under reduced pressure to obtain a yellow solid. After separation by column chromatography, intermediate 1 is obtained; wherein, the mass dosage is 2,4-dichloroquinazoline: isopropanol: p-phenylenediamine: DIPEA=280:10:90:0.7;
[0051] (2) the intermediate 1 was dissolved in tetrahydrofuran and stirred in an ice bath, p-fluorophenylacetyl chloride was added, triethylamine was added dropwise, then the temperature was raised to 30° C. to react for 1 hour, and concentrated under reduced pressure. After the crude product was separated by column chromatography Intermediate 2 was obtained; wherein, intermediate 1: tetrahydrofuran: p-fluorophenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com