Application of tangeretin in preparing oxidative stress protection product
An oxidative stress, citrus peeling technology, applied in the application, the function of food ingredients, medical preparations containing active ingredients, etc., to achieve the effect of increasing the ability to resist hydrogen peroxide stress damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
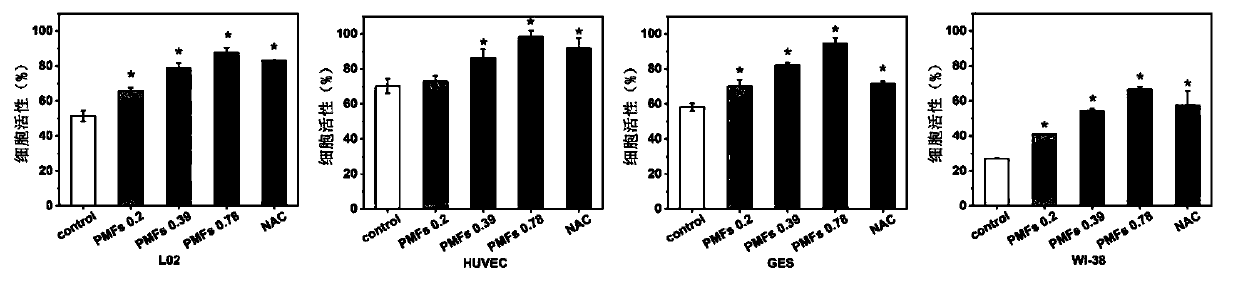
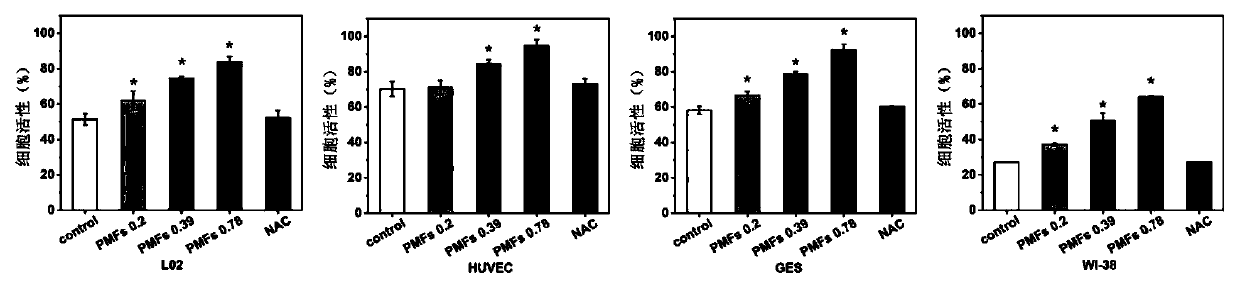
[0020] Example 1 Effect of Tangeretin on Cell Viability of Human Liver L02 Cells, Human Stomach GES Cells, Human Umbilical Vein Epithelial HUVEC Cells, and Human Fibroblast WI-38 Cells Injured by Hydrogen Peroxide Oxidative Stress
[0021] Human liver L02 cells, human gastric GES cells, human umbilical vein epithelial HUVEC cells, and human fibroblast WI-38 cells were cultured with RPMI-1640 containing 10% fetal bovine serum at 37°C and 5% CO 2 Incubate in a cell culture incubator, replace with fresh culture medium every other day, and passage once every 2-3 days. During the experiment, L02, GES, HUVEC, and WI-38 cells were seeded in 96-well plates. When the cells grow to 70-80% confluence, the original medium is discarded, and the drugs are added in groups. Set blank control group (without hydrogen peroxide treatment), DMSO solvent control group, N-acetylcysteine (NAC) control group (final concentration is 2mmol / L) and tangeretin treatment group (final concentration is 0.5...
Embodiment 2
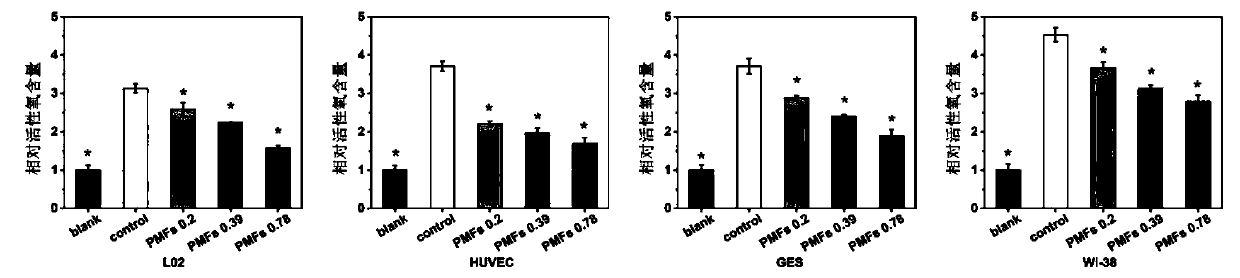
[0023] Example 2 Effects of Tangeretin on Intracellular Active Oxygen Content in Human Liver L02 Cells, Human Stomach GES Cells, Human Umbilical Vein Epithelial HUVEC Cells, and Human Fibroblast WI-38 Cells Injured by Hydrogen Peroxide Oxidative Stress
[0024] Human liver L02 cells, human gastric GES cells, human umbilical vein epithelial HUVEC cells, and human fibroblast WI-38 cells were cultured with RPMI-1640 containing 10% fetal bovine serum at 37°C and 5% CO 2Incubate in a cell culture incubator, replace with fresh culture medium every other day, and passage once every 2-3 days. During the experiment, L02, GES, HUVEC, and WI-38 cells were seeded in 96-well plates. When the cells grow to 70-80% confluence, the original medium is discarded, and the drugs are added in groups. Set up a blank control group (without hydrogen peroxide treatment), a DMSO solvent control group and different concentrations of tangeretin treatment groups (final concentrations were 0.5 μmol / L, 1 μm...
Embodiment 3
[0026] Example 3 Effect of Tangeretin on the Intracellular Antioxidative Enzyme Activity of Human Liver L02 Cells, Human Stomach GES Cells, Human Umbilical Vein Epithelial HUVEC Cells, and Human Fibroblast WI-38 Cells
[0027] Human liver L02 cells, human gastric GES cells, human umbilical vein epithelial HUVEC cells, and human fibroblast WI-38 cells were cultured with RPMI-1640 containing 10% fetal bovine serum at 37°C and 5% CO 2 Incubate in a cell culture incubator, replace with fresh culture medium every other day, and passage once every 2-3 days. During the experiment, L02, GES, HUVEC, and WI-38 cells were seeded in 96-well plates. When the cells grow to 70-80% confluence, the original medium is discarded, and the drugs are added in groups. Set up DMSO solvent control group and different concentrations of tangeretin treatment groups (final concentrations were 0.5 μmol / L, 1 μmol / L, 2 μmol / L, respectively). After acting for 6 hours, the culture medium containing tangereti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com