Use of quinazoline compounds and avastin in preparation of disease-prevention combined drugs
A quinazoline and compound technology, which can be used in drug combinations, neurological diseases, anti-tumor drugs, etc., can solve problems such as toxic and side effects, achieve good efficacy, prolong the time required for drug resistance, and prolong progression-free survival. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
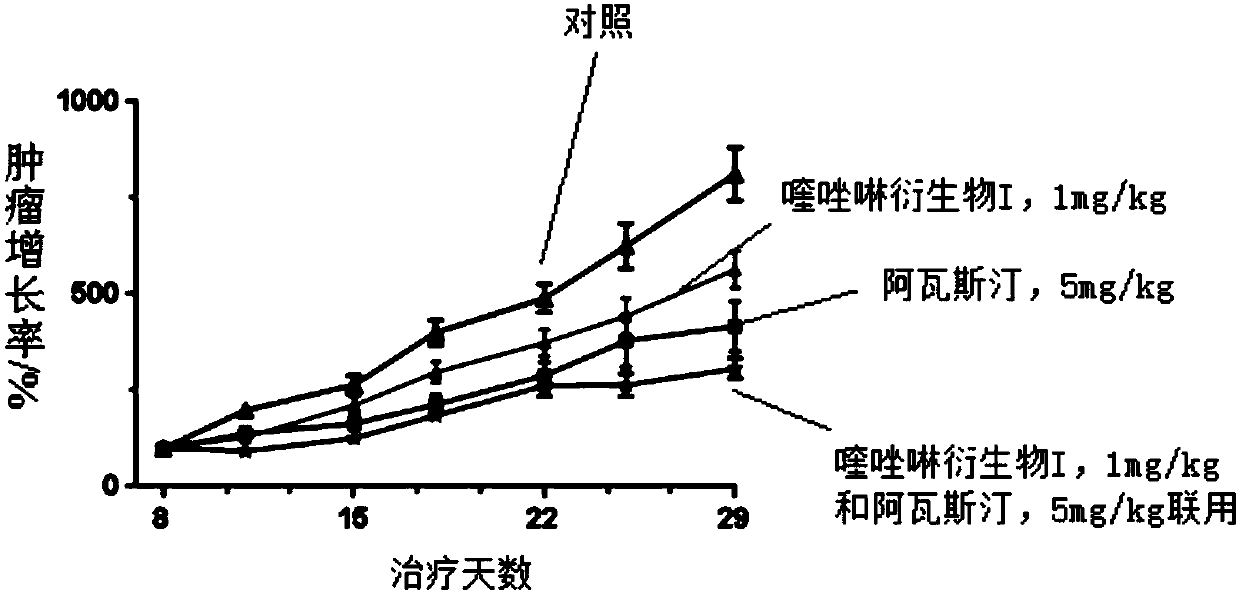
[0021] Inhibitory effect of Avastin-quinazoline derivatives (I) according to the present invention on xenograft tumor growth in non-small cell lung cancer PC-9 mice
[0022] Non-small cell lung cancer PC-9 cancer cells (5x 10 6 ), in 0.1ml of RPMI-1640andMatrigel (BD, cat.NO.356234) (1:1ratio) cell suspension, inoculated subcutaneously in the right wing of mice. After 8 days, use a vernier caliper to measure the diameter of the mouse transplanted tumor, and wait until the tumor grows to 150-200mm 3 Animals were then randomly grouped. Using the method of measuring tumor diameter, dynamically observe the antitumor effect of the tested drug. Tumor diameter was measured once every 3 days, and the weight of the mouse was also weighed at the same time as each measurement. The grouping and administration methods are as follows:
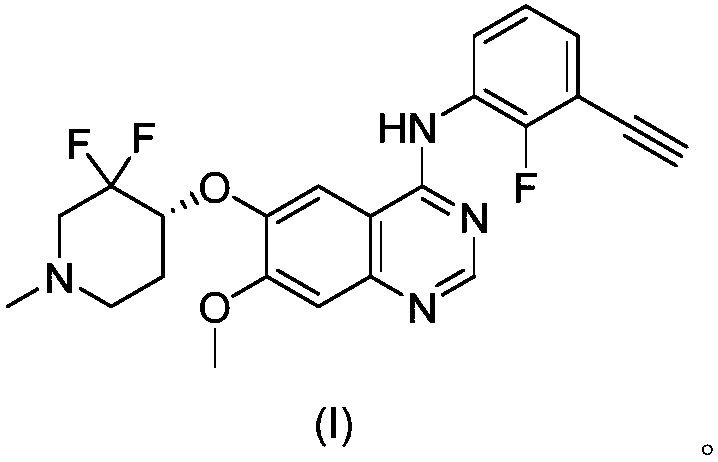
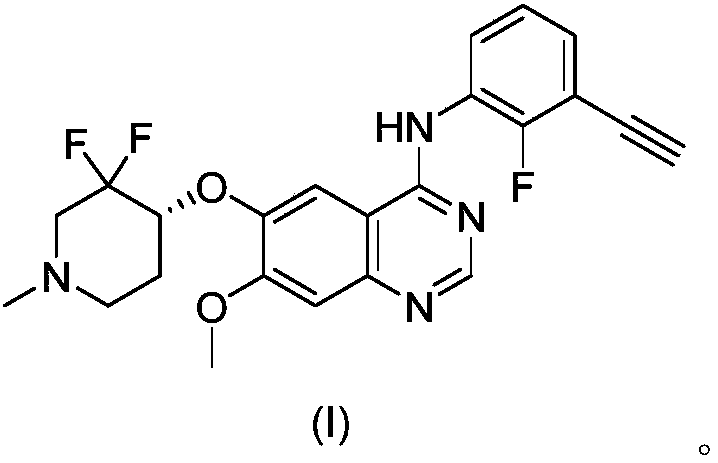
[0023] The Avastin group was injected intraperitoneally, 5 mg / kg, twice a week. The quinazoline derivative (I) group of the present invention was admin...
Embodiment 2
[0025] Drug efficacy of PC-9 tumor cell mouse subcutaneous animal model
[0026] like figure 1 As shown, in the drug efficacy experiment of PC-9 tumor cell mouse subcutaneous animal model, administration began on the eighth day after planting tumor cells, and the second group of quinazoline derivatives (I) single drug, 1 mg / kg, orally , twice a day and the third group of Avastin, single drug, intraperitoneal administration, twice a week compared with the first group of the control group (without drug group) all showed good inhibition of tumor growth, with statistically significant drug effects .
[0027]
[0028] The fourth group (quinazoline derivative (I) and Avastin composition) of the pharmaceutical composition of the present invention unexpectedly shows a synergistic effect, and improves the efficacy of a single drug compared with the second group and the third single drug group , and there was a statistically significant difference in efficacy. Compared with the bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com