Polypeptide-antigen conjugates with non-natural amino acids
A technology of unnatural amino acids and conjugates, applied in the field of polypeptide-antigen conjugates with unnatural amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
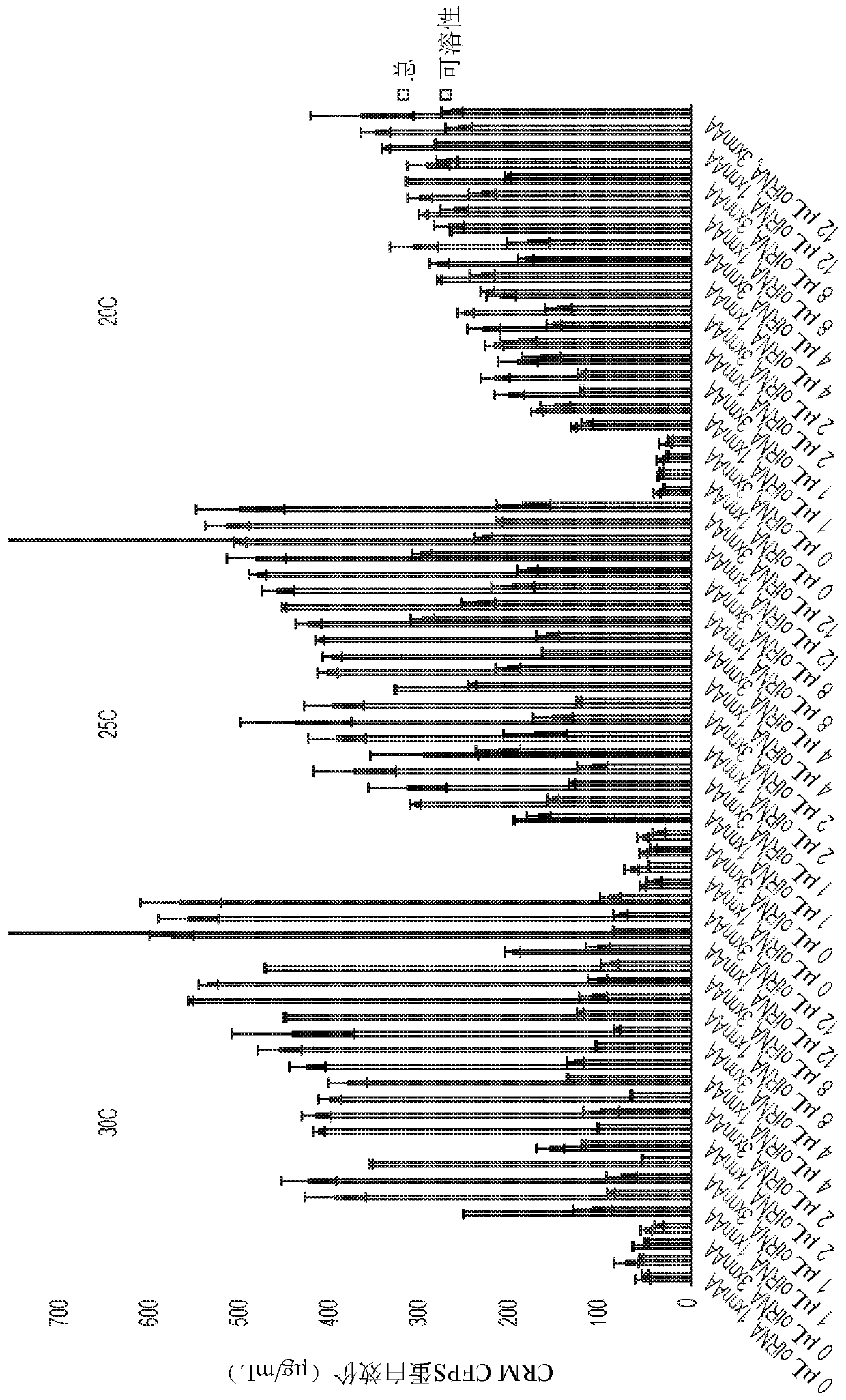
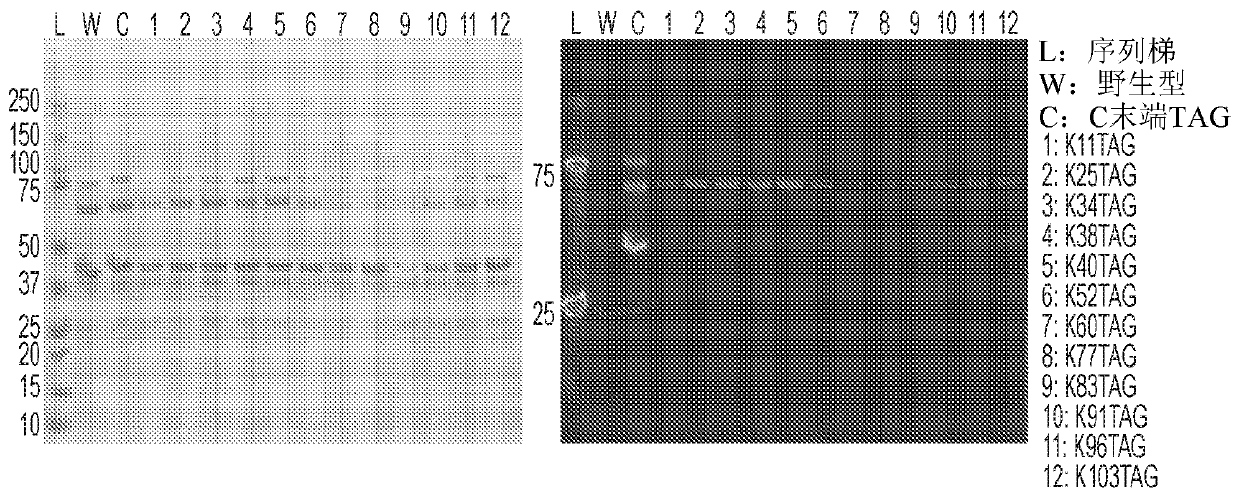
[0506] Example 1: Synthesis of single site eCRM fractions K11TAG, K25TAG, K34TAG, K38TAG, K40TAG, K52TAG, K60TAG, K77TAG, K83TAG, K91TAG, K96TAG and K103TAG
[0507] eCRM was expressed in cell-free protein synthesis (CFPS) extracts provided by Sutro Biopharma, Inc. (South San Francisco, Calif.). The characterization and preparation of such extracts are described in other publications; in this case, the extracts are usually as described in Zawada et al., 2011, "Biotechnol. Bioeng.", 108(7) , 1570-1578, using the following modifications from US2016 / 0257946: (1) Cell-free extracts were prepared from outer membrane protein T-sensitive RF-1 attenuated strains engineered to to overexpress E. coli DsbC; (2) prepare cell-free extracts from a similar RF-1 attenuated E. coli strain engineered to produce an orthogonal CUA-encoding tRNA for insertion at the amber stop codon Unnatural amino acids; (3) the cell-free extracts from (1) and (2) were mixed (85:15 ratio) and treated with 50 μM ...
example 2
[0514] Example 2: Design of multiple nnAA eCRMs
[0515] Multiple nnAA eCRM variants were selected as described in the detailed description above. Variants were synthesized by CFPS and tested as in Example 1.
[0516] Table 2: Various nnAA eCRM variants
[0517] Variants# K25 K34 K38 K40 K213 K215 K228 K245 K265 K386 K523 K527 1 √ √ √ √ √ √ 2 √ √ √ √ √ √ 3 √ √ √ √ √ √ 4 √ √ √ √ √ √ 5 √ √ √ √ √ √ 6 √ √ √ √ √ √ 7 √ √ √ √ √ √ 8 √ √ √ √ √ √ 9 √ √ √ √ √ √ 10 √ √ √ √ √ √ 11 √ √ √ √ √ √ 12 √ √ √ √ √ √ 13 √ √ √ √ √ √ 14 √ √ √ √ √ √ 15 √ √ √ √ √ √ 16 √ √ √ √ √ √ 17 √ √ √ √ √ √ 18 √ √ ...
example 3
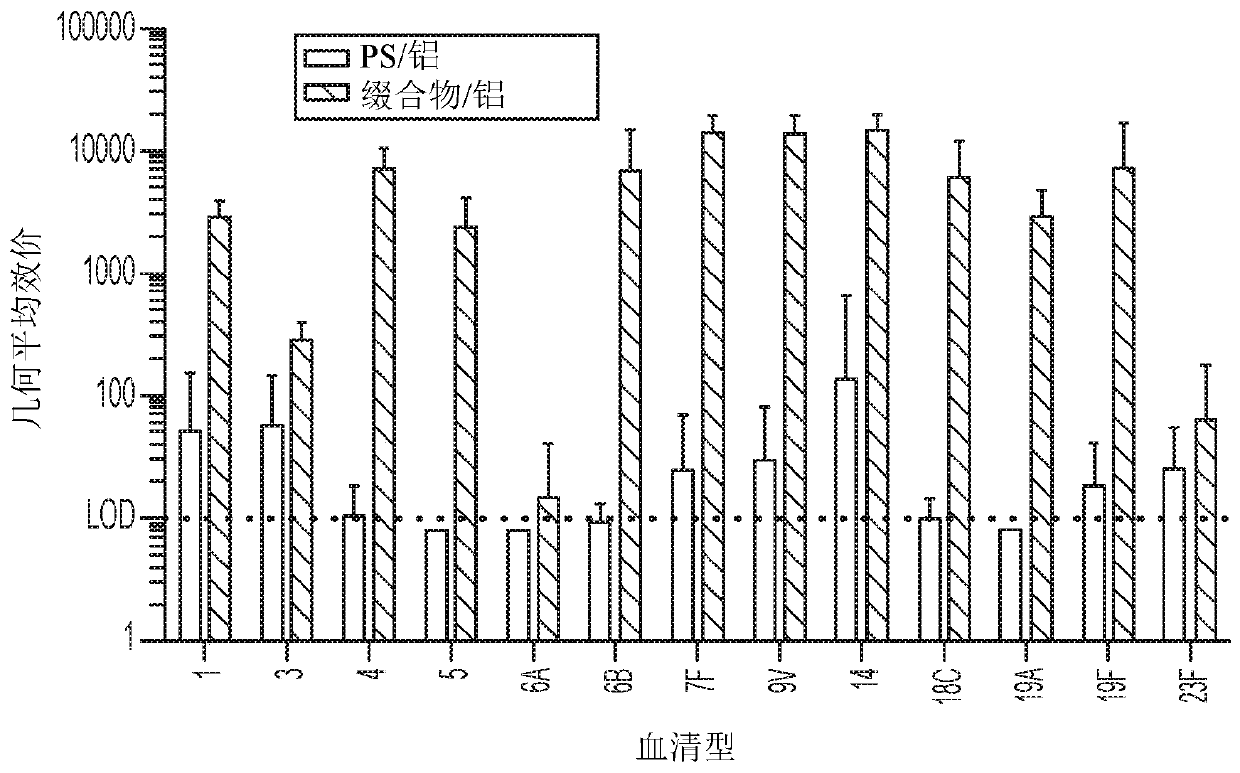
[0524] Example 3: Identification of T cell epitopes in Pfs25
[0525] According to the method described in Diethelm-Okita et al., " Journal of Infectious Diseases (J Infect Dis.) " 1997 February; 175 (2): 382-91, T Cell activation epitopes. Briefly, a peptide fragment consisting of 20 amino acids and overlapping by 5 amino acids was synthesized corresponding to the complete expression sequence of Pfs25. CD8+-depleted and CD4+-enriched human peripheral blood lymphocytes (PBL) were obtained from multiple subjects. PBLs were plated in triplicate and incubated with individual synthetic peptides spanning the sequence of Pfs25 serving as experimental stimuli. By having [ 3 Cultures of [H]-thymidine were pulsed to measure PBL proliferation in response to each peptide fragment and compared across cultures derived from different individuals. Fragments of Pfs25 that stimulate proliferation were identified as including T cell epitopes. Fragments that stimulated PBL proliferation fro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com