Method of manufacturing human antibody possessing bioreceptor synergist, antagonist and/or inverse synergist
A synergist and antagonist technology, applied in the field of manufacturing fully human antibodies, can solve problems such as clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the preparation of polypeptide antigen
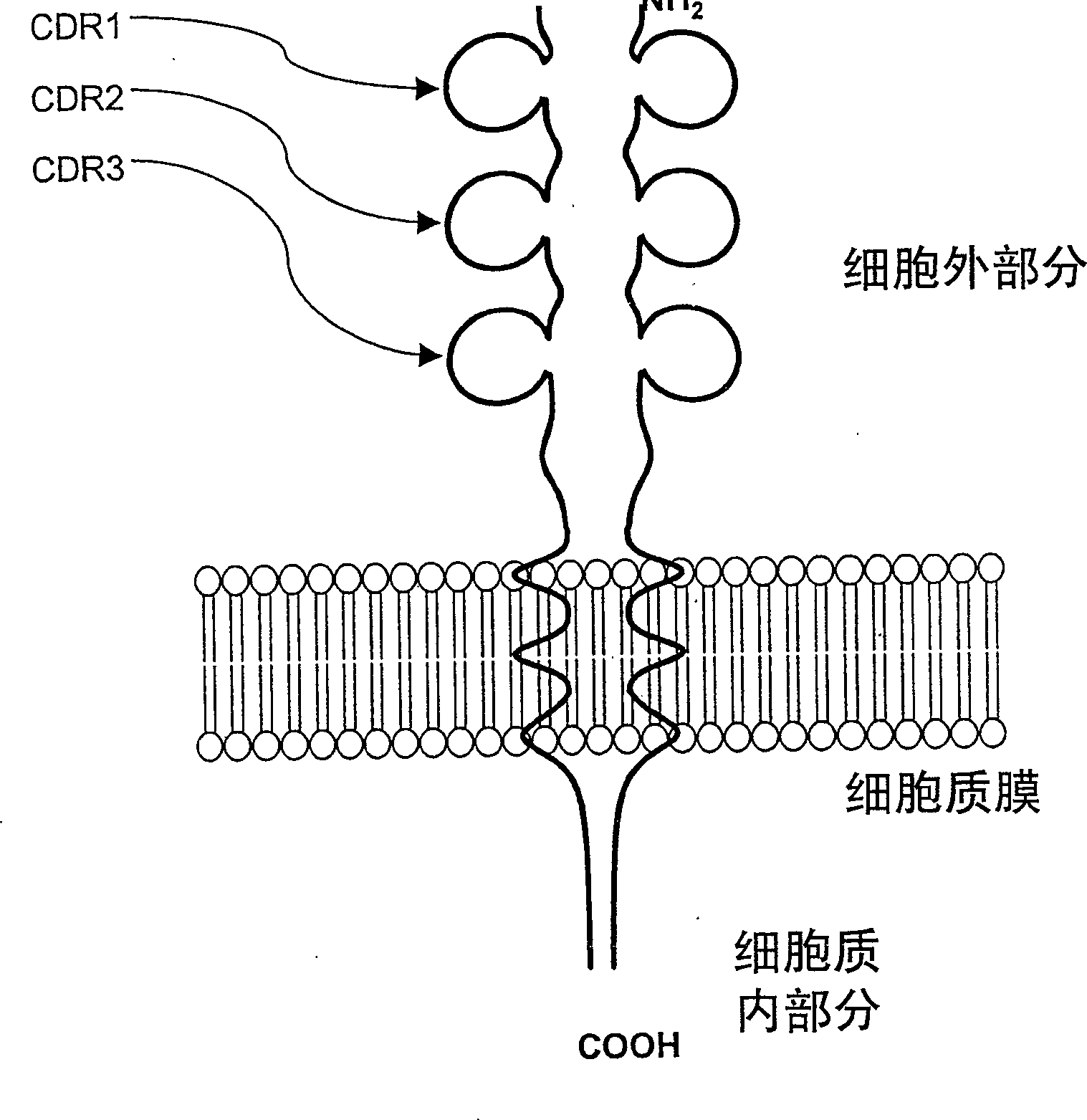
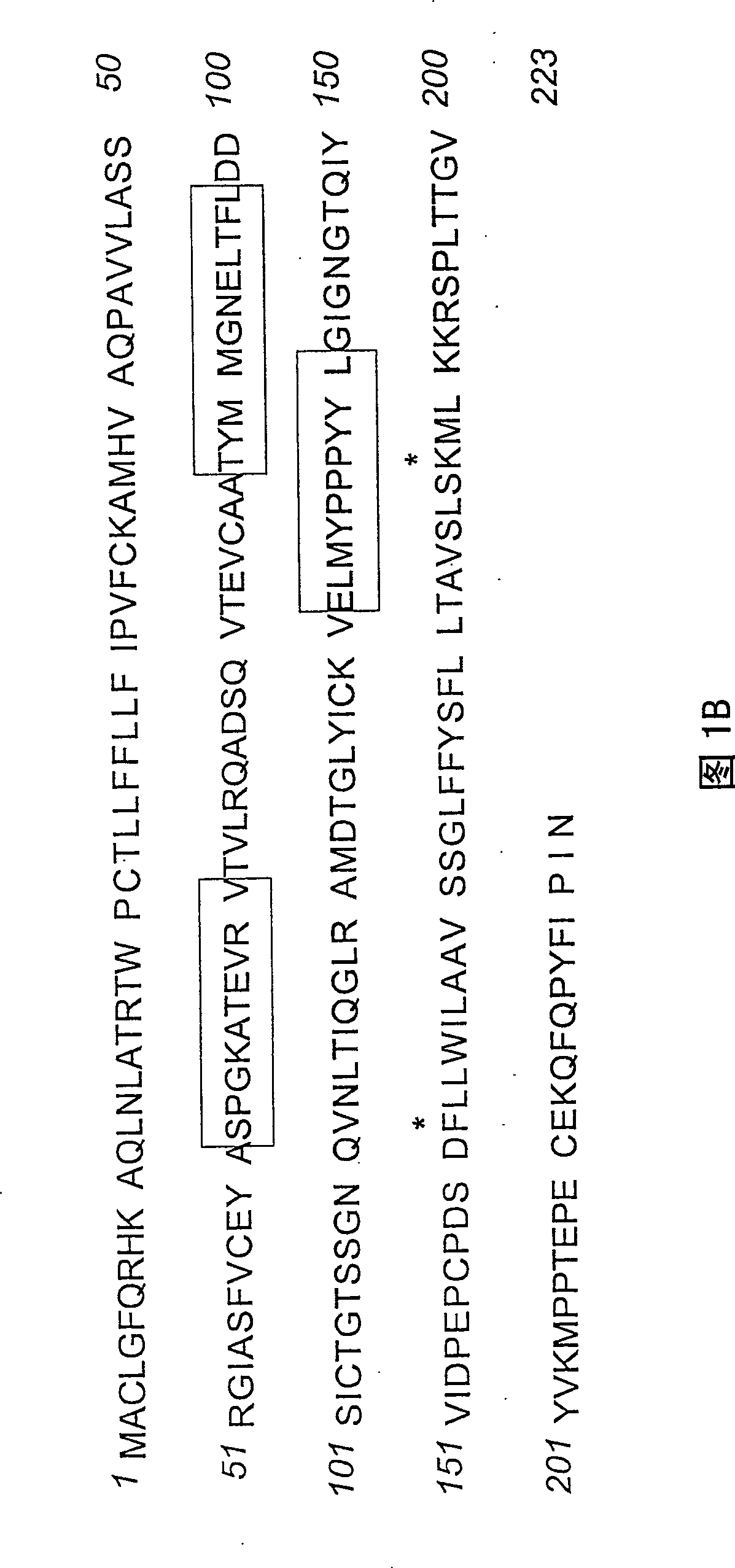
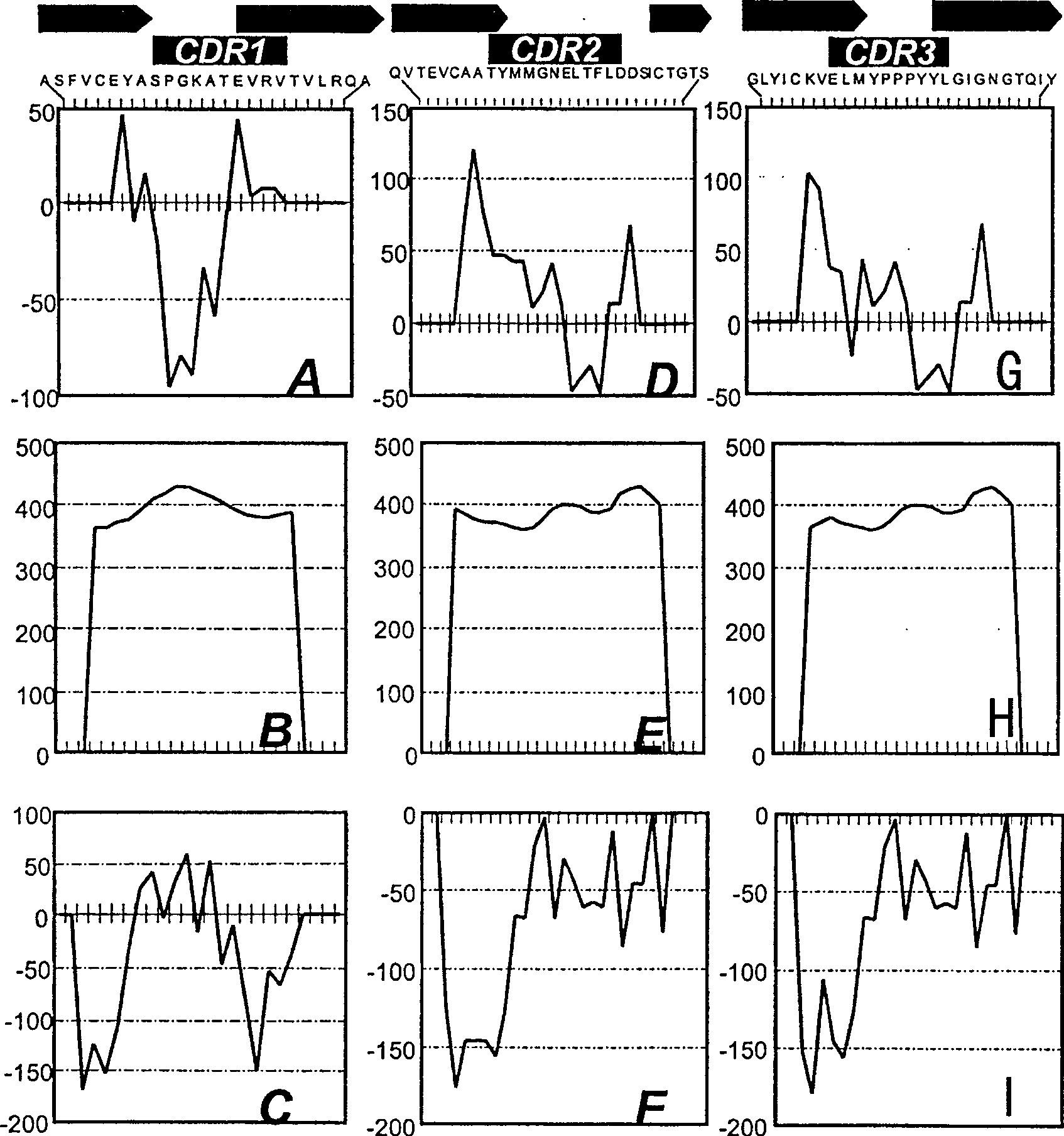
[0051] The overall structure of the human CD152 receptor is drawn in Figure 1A . The amino acid sequence corresponding to human CD152 is shown in Figure 1B. From the integration of information obtained from the scientific literature, an important relationship between the CDR3 region and native CD80 and CD86 ligands was thus established. Additional evidence suggests that amino acids located in the CDR1 region play a role in the interaction with CD80 / CD86. However, the synergist binding mechanism of CDR2 has not been fully explored so far.
[0052] To elicit antibodies that bind to the native protein, the polypeptides of the protein used must have properties similar to their conformation. Therefore, the complete sequences of CDR1, CDR2 and CDR3 regions were preserved for the design of synthetic immunogens. In addition, each CDR polypeptide portion was extended to conform to the aforementioned epitope pattern. To...
Embodiment 2
[0054] Example 2, Production of Anti-CD152 Human Antibody
[0055] Healthy blood donors were screened negative for HIV-1 / 2, HTLV-I / II, HCV, and HbsAg, and contained a normal amount of alanine transferase (ALT). The blood was from the Tainan Blood Donation Center of the Chinese Blood Foundation (Tainan, Taiwan). Peripheral blood mononuclear cells (PBMC) were separated in Ficoll-Paque (Amersham Biosiences) by density centrifugation (400xg); cells were then washed twice with PBS and collected by centrifugation at 100xg.
[0056] The obtained PBMCs were first magnetically labeled with CD45RO MACS microbeads (Miltenyi Biotec), and then separated with VarioMACS (Miltenyi Biotec). After magnetic calibration, cells are passed through a separation column, which is placed on a powerful permanent magnet. The matrix of the magnetic column can generate high-order magnetic field. Magnetically labeled cells remain in the column and unlabeled cells are filtered out. After the magnetic fie...
Embodiment 3
[0062] Example 3. Altering the ability of anti-CD152 antibody to induce apoptosis and cell differentiation and comparing with natural synergists
[0063] The binding sites of the different anti-CD152 human antibodies were identified by aligning synthetic polypeptides to primary alkylamine derivatized cellulose membranes (Rapp Polymere GmbH). In order to further evaluate the pharmacological effects of different anti-CD152 antibodies and a better natural synergist CD80, cell growth and PBMC culture of human peripheral lymphocytes stimulated with phytohemagglutinin in vitro were carried out. Briefly, prepare a flat-bottomed 96-well microtiter plate and add 50 μl of the cell suspension (10 5cells), 60 μl of PHA-containing medium (the final concentration of culture is 1.25 μg / ml, Amersham Biosciences AB), 40 μl of autologous plasma and 50 μl of RPMI-1640 medium containing anti-CD152 antibody or monomeric human CD80-muIg The concentration of fusion protein (Ancell) ranged from 0.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com