Dechlorination method of chlorinated aromatic compound (R1-Xm)
An aromatic compound, r1-xm technology, applied in the field of dechlorination of chlorinated aromatic compounds, can solve problems such as complex process and achieve the effect of safe catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
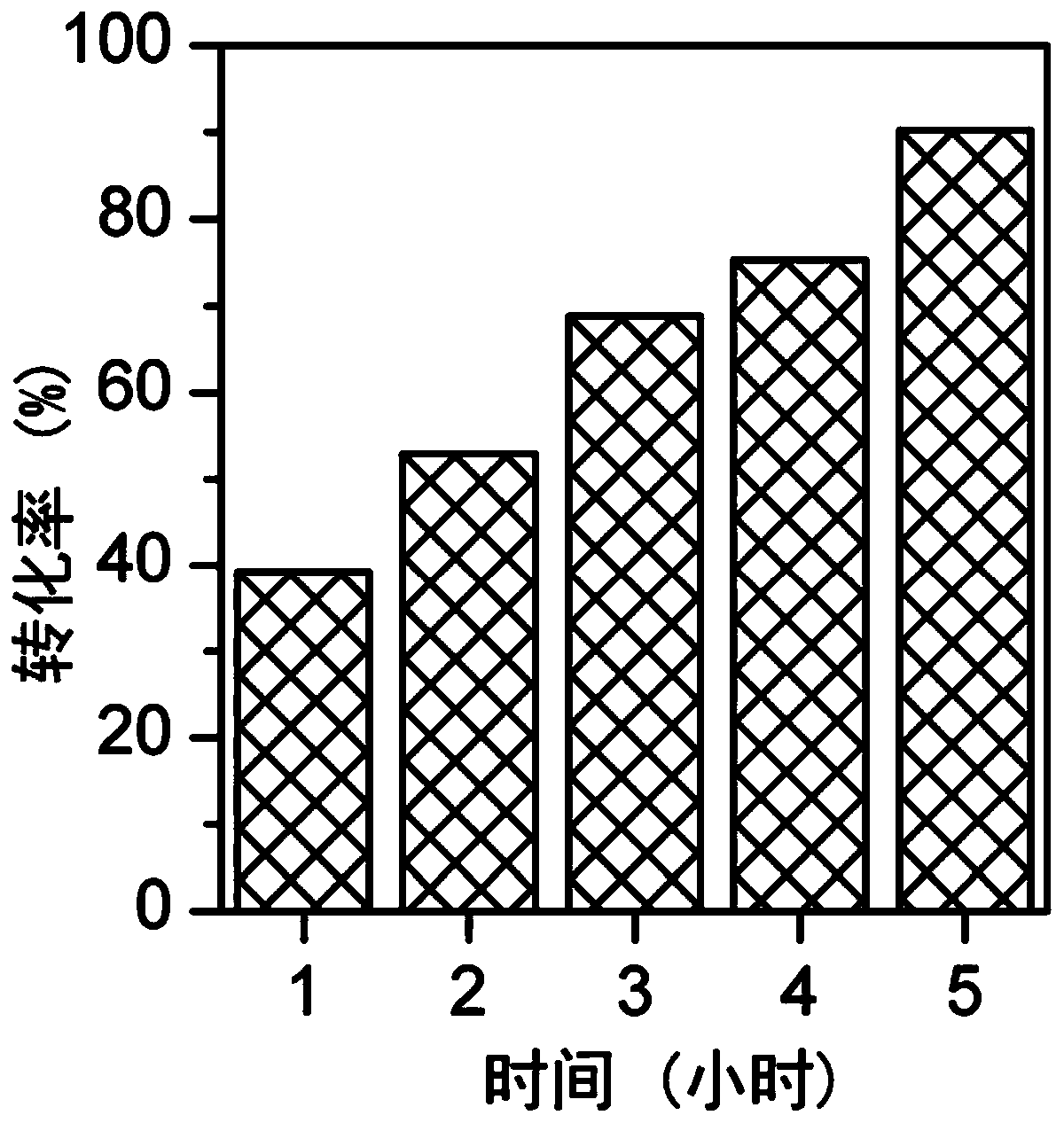
[0061] According to the dechlorination method provided by the present invention, 100 mL of methanol, 10 g of 1,2,3-trichlorobenzene, 2.5 g of 3% Pd / C catalyst, and 20 mL of 30% NaOH solution were added to a 500 mL autoclave. The aforementioned mixture was stirred evenly, hydrogenation reaction was carried out at 100° C., and time-tracking was carried out. like figure 1 As shown, the conversion rate of 1,2,3-trichlorobenzene reached 40% after 1 hour of reaction; after 5 hours of reaction, the conversion rate of 1,2,3-trichlorobenzene was 90%.
Embodiment 2-1~2-4
[0063] According to the dechlorination method provided by the present invention, the hydrodechlorination performance at different temperatures is evaluated. Take 10g of 1,2,3-trichlorobenzene, 3g of 3% Pd / C catalyst, and 30mL of 30% NaOH solution, and react for the same time. The results are shown in the table below: As the temperature increased, the conversion rate of the substrate continued to increase.
[0064] Table 1, the hydrodechlorination performance of the dechlorination method provided by the present invention at different temperatures
[0065]
Embodiment 3
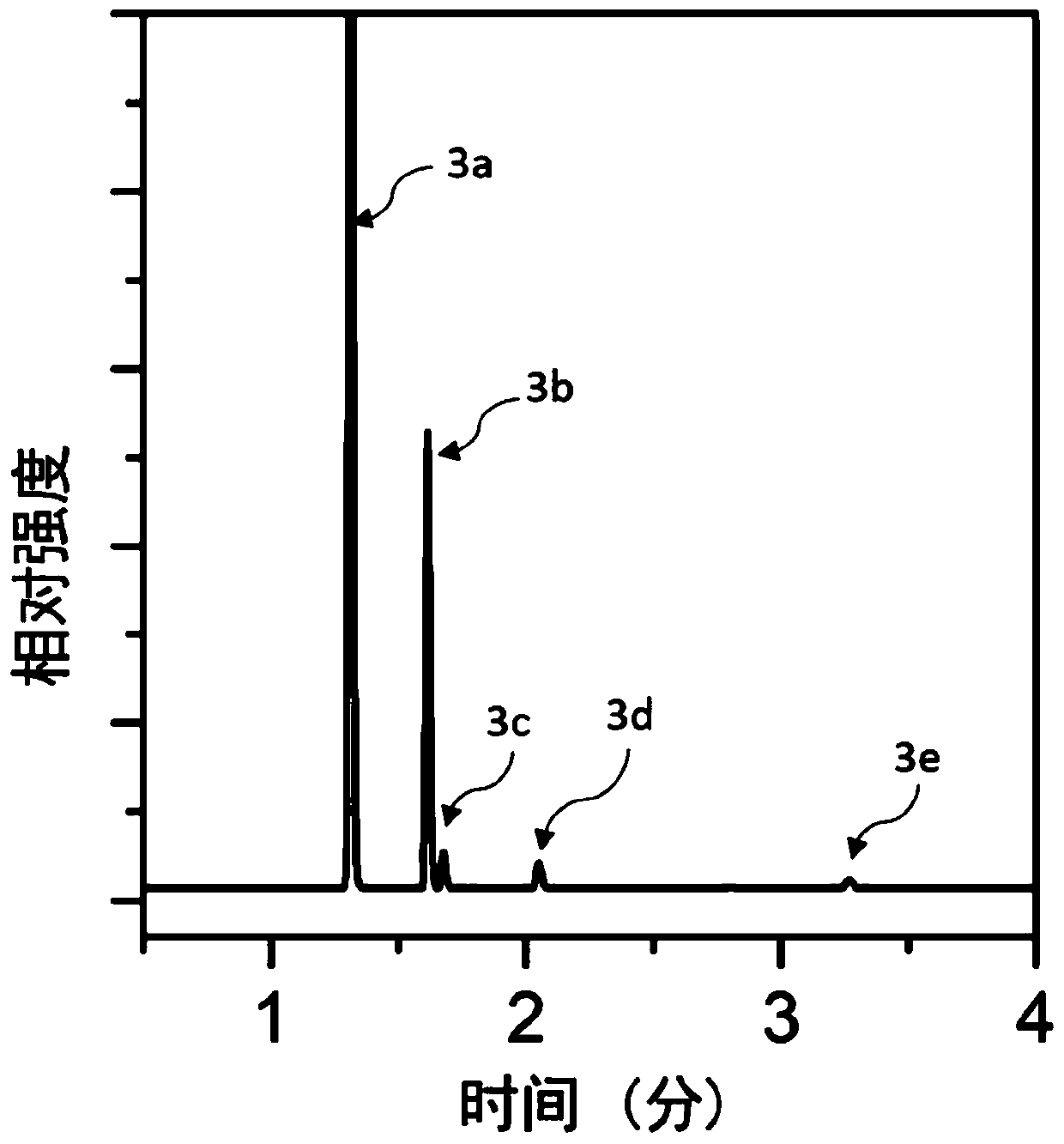
[0067] According to the dechlorination kit provided by the present invention, take 10 g of hexachlorobenzene as a substrate, 3 g of 3% Pd / C catalyst, 30 mL of 30% NaOH solution, and 100 mL of methanol solution, and react at 180° C. for 60 minutes. The product after the reaction was analyzed by gas chromatography, and the results were as follows: figure 2 As shown, the peaks 3a, 3b, 3c, 3d, and 3e from left to right in the figure represent methanol, benzene, chlorobenzene, o-dichlorobenzene, and hexachlorobenzene, respectively. The conversion rate of hexachlorobenzene is 99%, wherein the content of o-dichlorobenzene is 2%, the content of chlorobenzene is 2.54%, and the content of benzene is 94.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com