1,2-diol compound single crystal preparation method
A technology of compounds and diols, applied in the field of medicinal chemistry, can solve problems such as lack and easy occurrence of mixed crystal forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
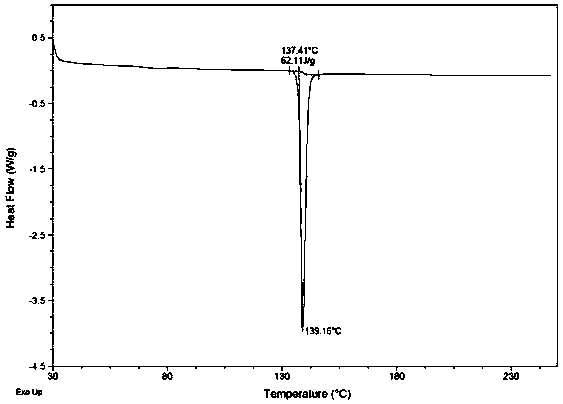
[0037] In a 100L reactor, add (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5 -(Propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopenta-1,2-diol 5.50kg, 27.5L of ethyl acetate, 110g of the compound of formula I (proportion 2.0%) was added, stirred and dissolved. Heat to 50-55°C, add dropwise 33L of n-heptane preheated to the corresponding temperature, control the temperature at 45-52°C during the process, keep it warm for 1h after dropping; then cool down to 10-15°C and keep it for 1h, filter, and use 0 -5°C Ethyl acetate / n-heptane 20kg (mass ratio 1:1) was washed 2-3 times and dried to obtain (1S,2S,3R,5S)-3-(7-{[(1R,2S) -2-(3,4-Difluorophenyl)cyclopropyl]amino}-5-(propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl )-5-(2-hydroxyethoxy)cyclopenta-1,2-diol crystal form 5.42 kg (yield 98.5%), with Figure 4 As shown in the DSC diagram (the tangent temperature is 137.41°C, the peak value is 139.16°C), the crystal form ...
Embodiment 2
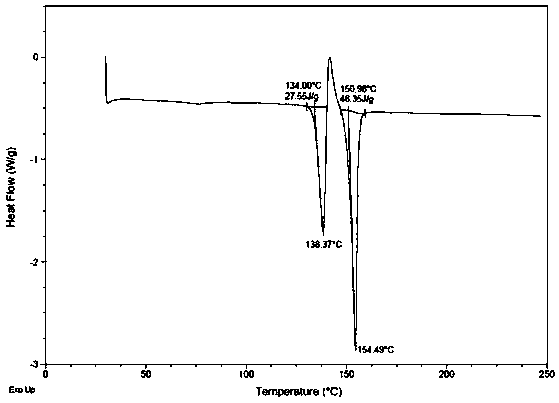
[0039] In a 100L reactor, add (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5 -(Propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopenta-1,2-diol 5.50kg, add 17L of ethyl acetate, then add 220g of the compound of formula I (proportion 4%), stir to dissolve. Heat to 50-55°C, add dropwise 38L of n-hexane preheated to the corresponding temperature, keep it at 45-52°C for 1h after dropping; then cool down to 10-15°C and keep it for 1h, filter, and use Wash with 20kg of n-hexane (mass ratio 1:1), and dry to obtain (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl) ring Propyl]amino}-5-(propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopenta -1,2-diol crystal form 5.39 kg (98.0% yield), with Figure 4 As shown in the DSC figure, the crystal form has the following figure 1 The XRD diffraction pattern shown proves that it is (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino} -5-...
Embodiment 3
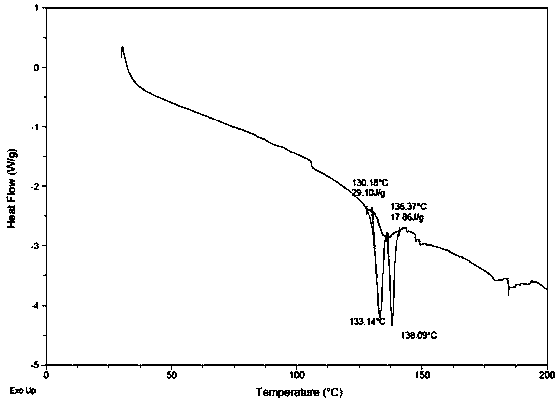
[0041] In a 100L reactor, add (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5 -(Propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopenta-1,2-diol 5.50kg, add 17L of ethyl acetate, then add 5.5g of the compound of formula I (proportion 0.1%), stir to dissolve. Heat to 50-55°C, add dropwise 38L of n-hexane preheated to the corresponding temperature, keep it at 45-52°C for 1h after dropping; then cool down to 10-15°C and keep it for 1h, filter, and use Wash with 20kg of n-hexane (mass ratio 1:1), and dry to obtain (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl) ring Propyl]amino}-5-(propylmercapto)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopenta -1,2-diol crystal form 5.40 kg (yield 98.2%), with Figure 6 As shown in the DSC spectrum, the crystal form has image 3 The XRD diffraction pattern shown proves that it is (1S,2S,3R,5S)-3-(7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino} -5-(Propylmer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com