Composition for treating neonatal HIE
A composition, neonatal technology, applied in the directions of microorganisms, drug combinations, biochemical equipment and methods, etc., can solve the problems of rejection, high production cost, storage and transportation limitations, etc., to achieve improved therapeutic efficacy, excellent therapeutic effect, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Induction of enhanced efficacy by treatment of stem cells with thrombin
[0064] 1-1. Thrombin-induced exosome secretion
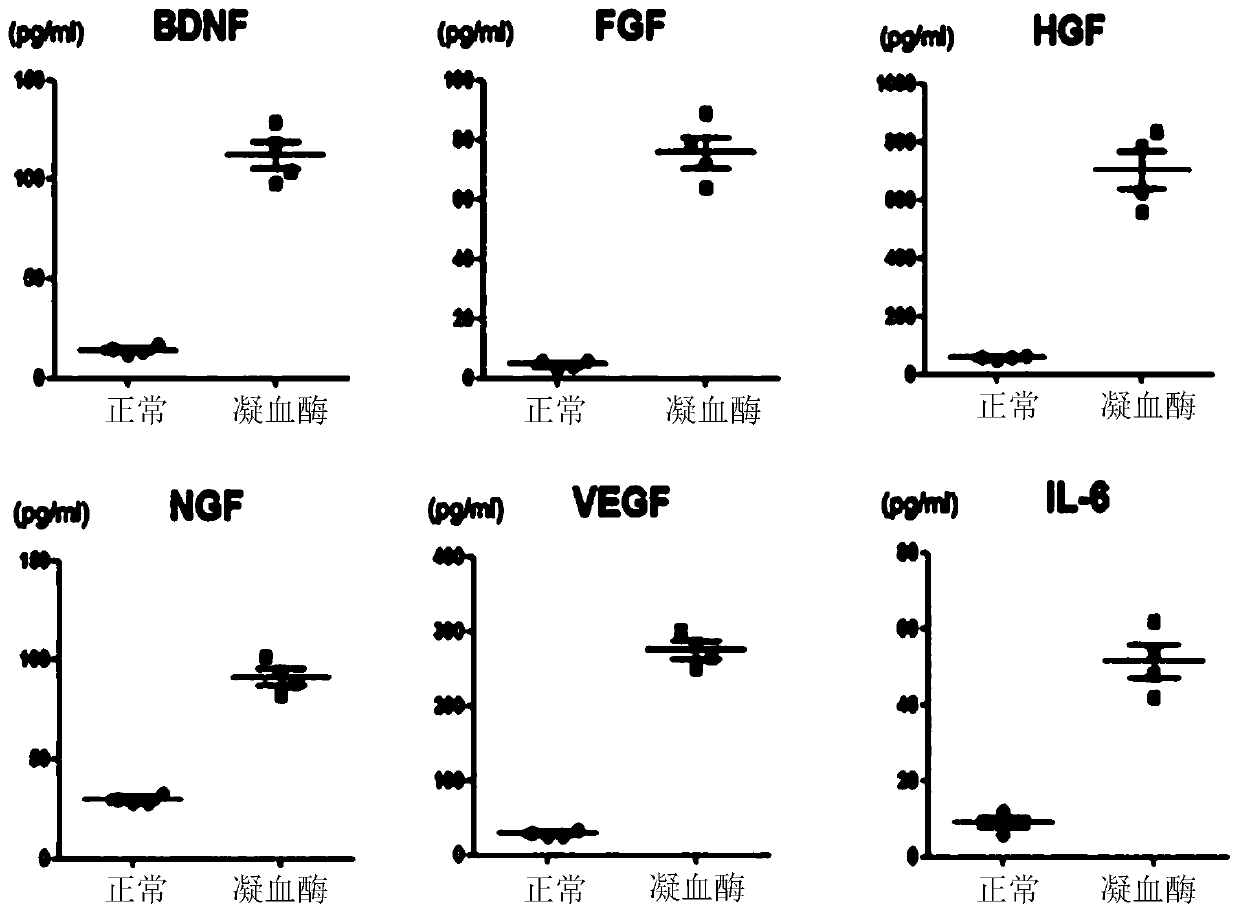
[0065] Human umbilical cord blood-derived mesenchymal stem cells (3×10 5 ) were inoculated on 60-mm Petri dishes (OrangeScientific cat#4450200) and cultured for 1 week. After confirming that the cells were saturated and proliferating in the culture dish, the medium was replaced with a serum-free medium (MEMα medium) (diluted 50 units / mL thrombin (REYON Pharmaceutical.Co., LTD) in a serum-free medium), and the cells were After culturing for another 6 hours, thrombin-treated stem cells were obtained.
[0066] Then, in order to confirm whether exosome secretion in mesenchymal stem cells was activated by thrombin treatment, the process of exosome secretion was confirmed by transmission electron microscope (TEM) images. The result is as figure 1 As shown, it can be seen that thrombin stimulation induces exosome secretion.
[0067] Alterna...
Embodiment 2
[0074] Example 2: Construction of an in vitro model of neonatal HIE
[0075] After 18.5 days of pregnancy, the lower abdomen of SD rats was dissected to remove the fetuses, and then the whole brains were carefully extracted. From the extracted brain tissue, only the cerebral cortex was carefully isolated, separated into individual cells using a pipette, and then cultured in neuronal cell culture solution. After 10 days of culture, cells were exposed to glucose-free medium under 1% hypoxic conditions (oxygen and glucose deprivation (OGD)) for 60 minutes to establish an in vitro model of neonatal HIE.
Embodiment 3
[0076] Example 3: In vitro inhibitory effect of thrombin-treated stem cells on neuronal cell death
[0077] The in vitro neonatal HIE model constructed in Example 2 was treated with the thrombin-treated stem cells obtained in Example 1, and then the nerve cell viability was evaluated by MTT assay.
[0078] The result is as Figure 4As shown, in the in vitro HIE model (OGD), neuronal cell death was significantly increased due to oxygen / glucose deprivation compared with normal neuronal cells (NC), while compared with non-thrombin-treated stem cell groups (OGD+naive MSC) or Neuronal cell death was considerably affected in the “thrombin-pretreated stem cell-treated in vitro neonatal HIE group (OGD+MSC_thrombin-pre)” compared to the hypoxic-preconditioned stem cell group (OGD+MSC_thrombin-pre) Large inhibition, proving that thrombin-treated stem cells exhibited the most excellent neuronal cell protection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com