Imidazole carbene ligand with amide remote basic functional group as well as synthesis method and application thereof
A technology of carbene ligand and synthesis method, which is applied in the directions of organic compound/hydride/coordination complex catalyst, gold organic compound, chemical instrument and method, etc. Obstacles and other problems, to achieve the effect of simple and efficient response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
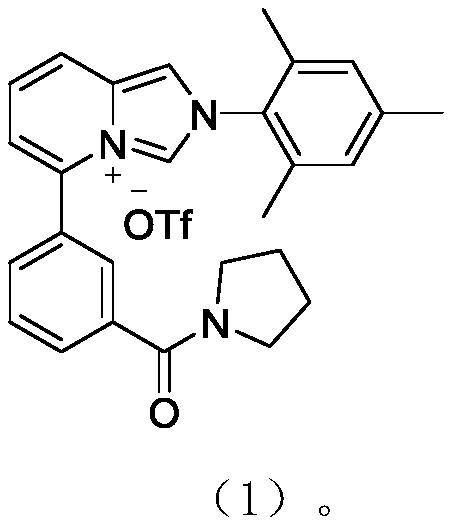
[0042] Imidazole carbene ligand, which is the compound described in formula (1)
[0043]
[0044] The compound is prepared as follows:
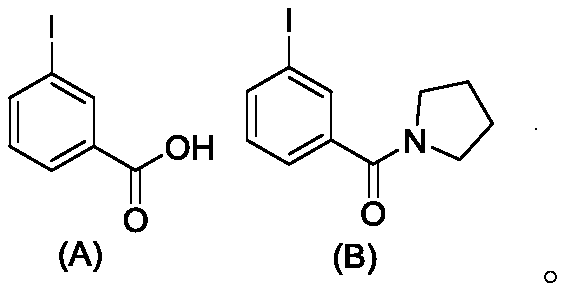
[0045] Step 1: Dissolve 3-iodobenzoic acid (A) (10 mmol) in 50 mL of dichloromethane, add oxalyl chloride (2.5 equiv) and 3 drops of N,N-dimethylformamide and stir at room temperature for 4 hours. The mixture was rotovaped and dried in vacuo to give the product 3-iodobenzoyl chloride. Under nitrogen protection, 3-iodobenzoyl chloride was dissolved again in 50 mL of dichloromethane and cooled in an ice bath. Will contain 15mmol tetrahydropyrrole (1.5equiv) and 20mmol Et 3 A solution of N(2 equiv) in 10 mL of dichloromethane was slowly added dropwise, then the reaction mixture was stirred at room temperature. After 1 hour, add 50mL of water and 100mL of dichloromethane, separate and dry the organic phase, spin dry, use a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1–2:1 as the eluent, and the column Chrom...
Embodiment 2
[0070] Synthesis of Imidazole Carbene Gold Complexes
[0071]
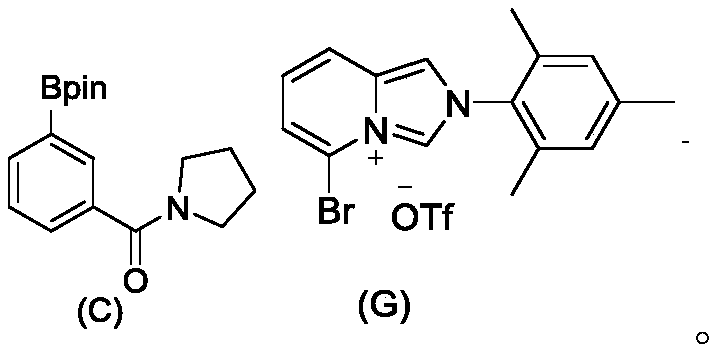
[0072] Under nitrogen protection, add 1 mmol of imidazole carbene ligand with amide remote basic functional group represented by formula (1) into 5 mL of redistilled tetrahydrofuran, and add 1.2 mmol of lithium bistrimethylsilylamide (1.2 equiv) at -78 °C , stirred for half an hour, added 0.95mmol dimethyl sulfide gold chloride (0.95equiv), stirred for 16 hours, spin-dried and passed through the column, and the mixed solution of dichloromethane:methanol with a volume ratio of 40:1-20:1 As the eluent, the eluate containing the target product is collected to obtain the carbene gold catalyst (2).
[0073] Pale yellow solid, yield 50%, detection result is as follows:
[0074] 1 H NMR (500MHz, DMSO) δ8.06(s, 1H), 7.76–7.72(m, 2H), 7.69–7.65(m, 2H), 7.54(t, J=7.7Hz, 1H), 7.16(dd, J=9.2,6.7Hz,1H),7.07(s,2H),6.74(dd,J=6.6,1.1Hz,1H),3.69(dd,J=28.3,6.4Hz,2H),3.45(t,J =6.9Hz, 2H), 2.31(s, 3H), 1.93(d, J=4.9Hz, 6H), 1....
Embodiment 3
[0076] Carbene gold catalyst (2) catalyzes the reaction of phenylacetylene and diphenylphosphonic acid to generate phenyl vinyl ether.
[0077]
[0078] Under nitrogen protection, 0.006mmol carbene gold catalyst (3mol%), 0.012mmol bistrifluoromethanesulfonate imide silver salt (6mol%), 0.2mmol diphenylphosphonic acid, 0.4mmol phenylacetylene in 1,2-dichloro In ethane, react at 50° C. for 12 hours to obtain the phenyl vinyl ether product with a yield of 67%.
[0079]
[0080] Pale yellow solid, the test results are as follows:
[0081] 1 H NMR (500MHz, CDCl3) δ7.93-7.88(m, 4H), 7.61-7.53(m, 4H), 7.49-7.45(m, 4H), 7.38-7.34(m, 3H), 5.23(dd, J =2.8,2.2Hz,1H),5.18(dd,J=2.9,1.9Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com