Piperlongumine derivative as well as preparation method and application thereof
A technology for longinamide and derivatives, which is applied in the field of longinamide derivatives and their preparation, can solve problems such as not obtaining clear conclusions, and achieve the effects of high yield and simple synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A method for preparing a perylene amide derivative as described in formula (I-1), including any mixture of these forms or a pharmaceutically acceptable salt thereof, comprising the following steps:
[0045] a. Using 3-hydroxyl-4-nitrobenzaldehyde and propyne bromide as raw materials to prepare a product 1 with a propynyl group;
[0046] b. react product 1 with malonic acid and precipitate with dilute hydrochloric acid to prepare product 2 with chloroacrylic acid groups;
[0047] c. reacting the product 2 with oxalyl chloride, and reacting the resulting product with a chlorinated seven-membered lactam ring to prepare a perylene amide derivative as shown in formula (I-1).
[0048] specifically:
[0049] (1) Dissolve 3-hydroxy-4-nitrobenzaldehyde and Propargyl bromide in DMF (dimethylformamide), add potassium carbonate (K 2 CO 3 ), after several hours of reaction at room temperature, 5 times the volume of purified water was added and extracted with ethyl acetate. The o...
Embodiment 1
[0076] Embodiment 1: the preparation of the perylene amide derivative as shown in formula (I-1)
[0077] Dissolve 1.07g (1.0 equivalent) of 3-hydroxy-4-nitrobenzaldehyde and 0.9g (1.2 equivalent) of propyne bromide in 10mLDMF, add 0.2g (2.0 equivalent) of potassium carbonate, react at room temperature for 4 hours and add 5 times volume of purified water and extracted with ethyl acetate. The organic layers were combined and washed with saturated brine, dried over anhydrous sodium sulfate, mixed and loaded on the column, and the product was eluted with ethyl acetate:petroleum ether 1:4 to obtain 1.3 g of product 1 as a yellow solid, with a yield of 100%.
[0078] The obtained product 1 was dissolved in 8 mL of pyridine, 1.2 g (1.8 equivalents) of malonic acid and a catalytic amount of piperidine were added and reacted at 60° C. for 4 hours. TLC monitored until the raw material was consumed, and 1 mol / L dilute hydrochloric acid was added until the precipitate was no longer preci...
Embodiment 2
[0081] Embodiment 2: as the perylene amide derivative shown in formula (II-1) (R 3 For the preparation of nitro)
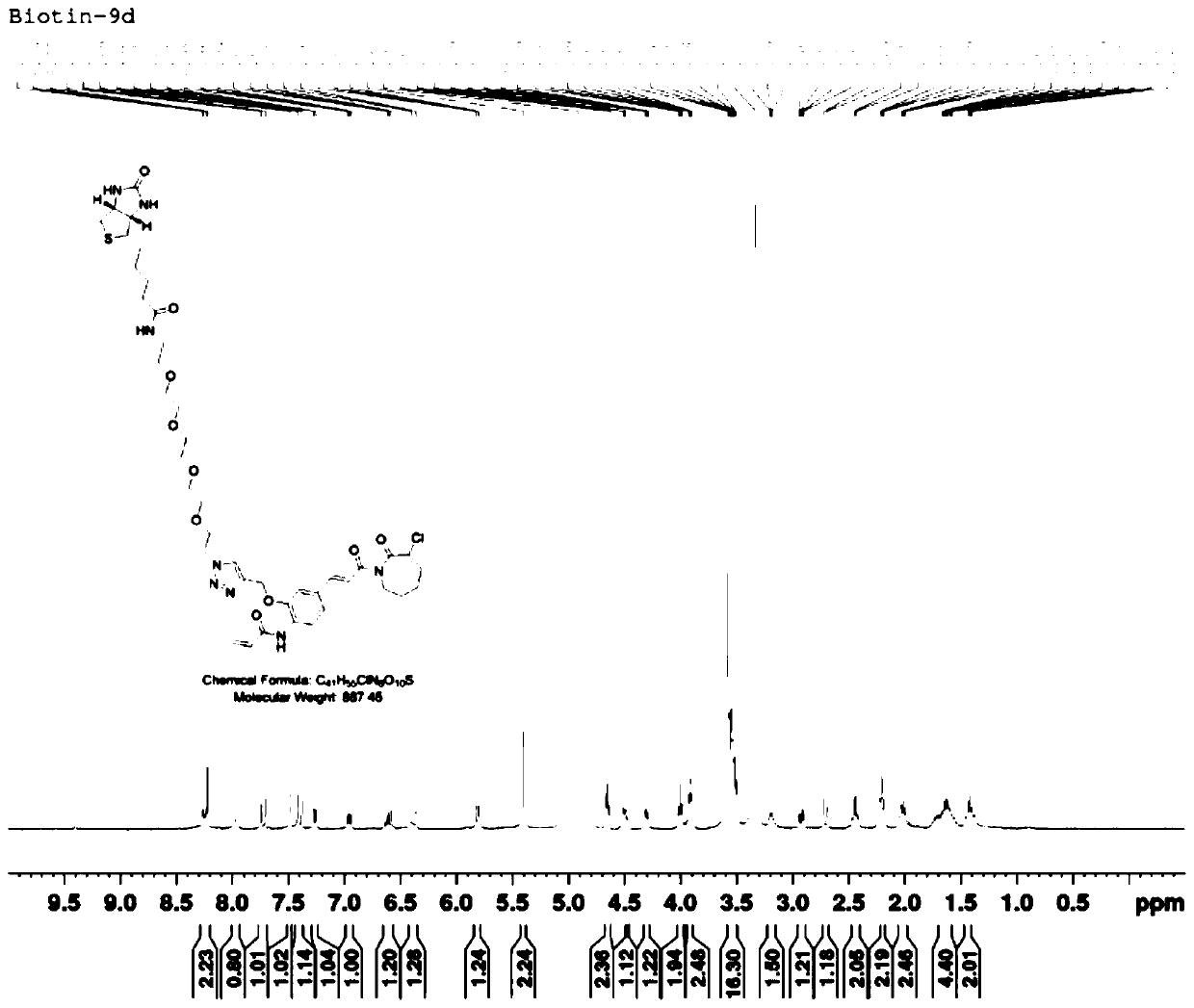
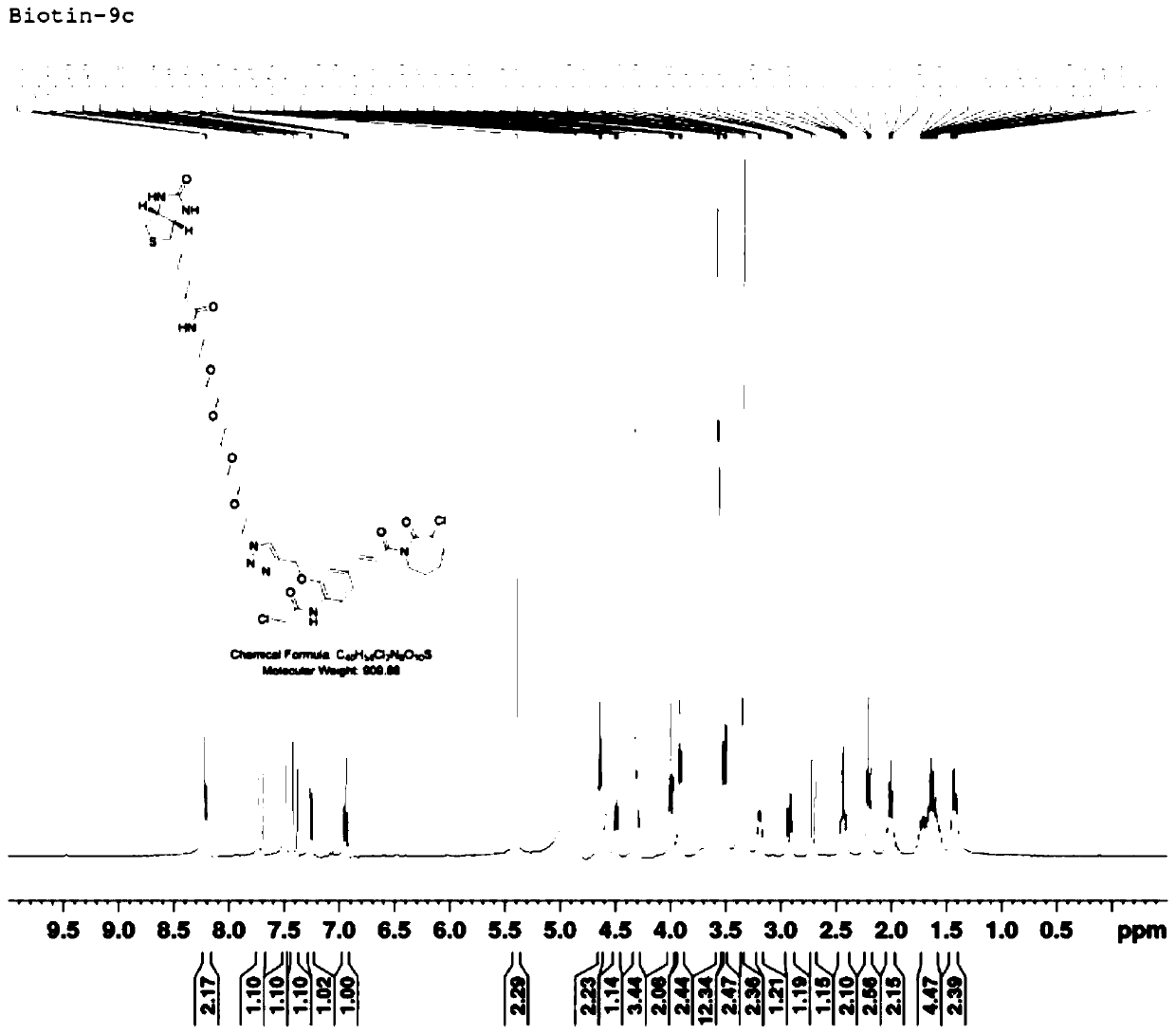
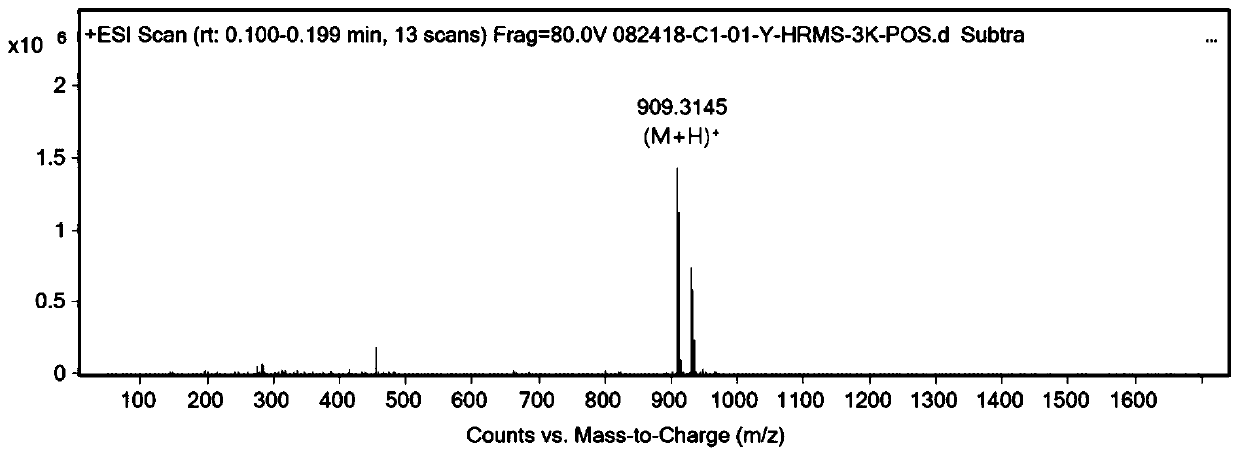
[0082] Dissolve 30 mg of product (I-1) in 5 mL of tert-butanol, add 8 mg (1.0 equivalent) of copper sulfate, add 30 mg (0.8 equivalent) of biotin-PEG4-azide, and protect with nitrogen. Dissolve 25 mg (3.0 equivalents) sodium vitamin C in 5 mL of pure water, slowly drop it into the reaction flask, react at room temperature for 4 hours, TLC monitors that the raw material is dry, add 5 times the volume of pure water and extract with ethyl acetate. The organic layers were combined and washed with saturated brine, dried over anhydrous sodium sulfate, and separated on a thick silica gel preparative plate with dichloromethane / methanol 10:1 to obtain the crude product. D., s-5m), the mobile phase is acetonitrile / water 75:25, the flow rate: 2mL / min, and 6 mg of light yellow oily liquid is obtained, which is the perylene amide derivative shown in formula (II-1) (R 3 is n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com