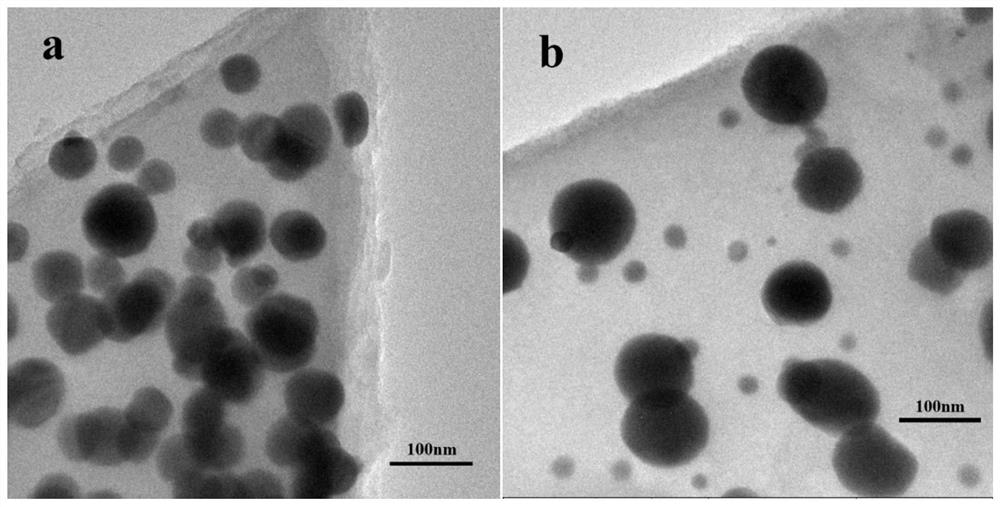
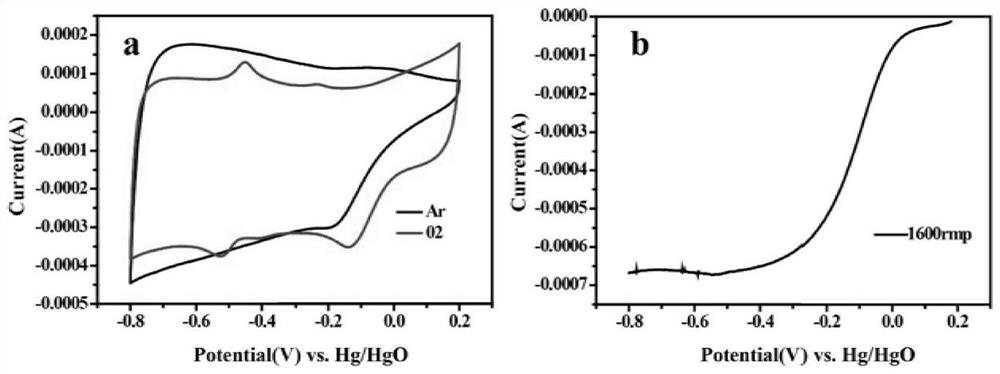
A kind of preparation method of carbon supported nano-silver catalyst
A nano-silver and catalyst technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of undeveloped catalysts, achieve excellent ORR performance, low price, and wide-ranging sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 70.2 mg (0.2 mmol) of tris(tetraaminophenyl) benzene and 35 mL of dichloromethane into a 100 mL round bottom flask, stir magnetically at room temperature until completely dissolved; weigh 102 mg (0.6 mmol) of AgNO 3 The solid was dissolved in 10 mL of dichloromethane, and was added dropwise to the above-mentioned round bottom flask at a uniform speed; after the dropwise addition was completed, the reaction was stirred at room temperature for 12 hours, and the precipitate was obtained by centrifugation. The obtained precipitate was vacuum-dried in a constant-temperature oven at 60° C. for 12 hours to obtain a polymer metal complex.
[0030] Put 0.2g of the polymer metal complex obtained in the above steps in a muffle furnace, and in an Ar gas atmosphere, heat up to 850°C at a rate of 5°C / min for calcination for 120min, and cool naturally to room temperature to obtain an Ag loading of 1eq carbon-supported silver nanocatalysts. (Initial potential: 0.92V; half-wave pot...
Embodiment 2
[0032] Add 70.2mg (0.2mmol) of tris(tetraaminophenyl)benzene and 35mL of dichloromethane into a 100mL round bottom flask, stir magnetically at room temperature until completely dissolved; weigh 153mg (0.9mmol) of AgNO 3 The solid was dissolved in 18 mL of dichloromethane, and was added dropwise to the above-mentioned round bottom flask at a uniform speed; after the dropwise addition was completed, the reaction was stirred at room temperature for 12 hours, and the precipitate was obtained by centrifugation. The obtained precipitate was vacuum-dried in a constant-temperature oven at 60° C. for 12 hours to obtain a polymer metal complex.
[0033] Put 0.2 g of the polymer metal complex obtained in the above steps in a muffle furnace, and in an Ar gas atmosphere, heat up to 850 °C for 120 min at a heating rate of 5 °C / min, and then cool naturally to room temperature to obtain an Ag loading of 1.5 eq's carbon-supported nanosilver catalyst. (Initial potential: 0.93V; half-wave poten...
Embodiment 3
[0035] Add 58mg (0.2mmol) of tris(tetraaminophenyl)amine and 29mL of chloroform into a 100mL round bottom flask, stir magnetically at room temperature until completely dissolved; weigh 102mg (0.6mmol) of AgNO 3 The solid was dissolved in 10 mL of chloroform, and was added dropwise to the above-mentioned round bottom flask at a uniform speed; after the dropwise addition was completed, the reaction was stirred at room temperature for 12 hours, and the precipitate was obtained by centrifugation. The obtained precipitate was vacuum-dried in a constant-temperature oven at 60° C. for 12 hours to obtain a polymer metal complex.
[0036] Put 0.2g of the polymer metal complex obtained in the above steps in a muffle furnace, and in an Ar gas atmosphere, heat up to 850°C at a rate of 5°C / min for calcination for 120min, and cool naturally to room temperature to obtain an Ag loading of leq carbon-supported silver nanocatalysts. (Initial potential: 0.91V; half-wave potential: 0.70V)
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com