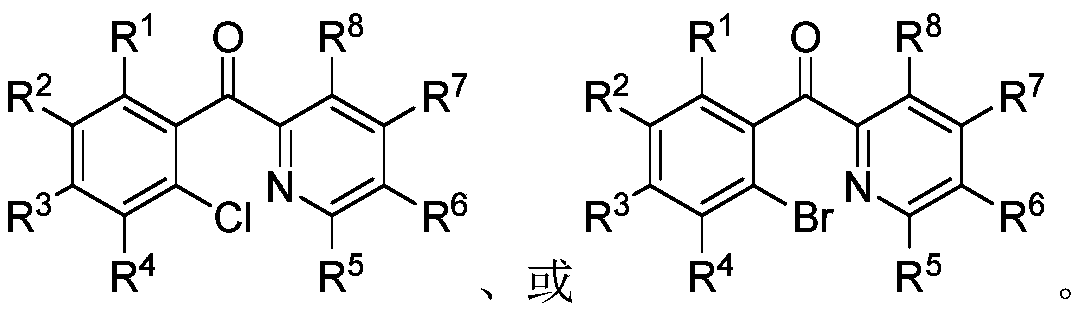
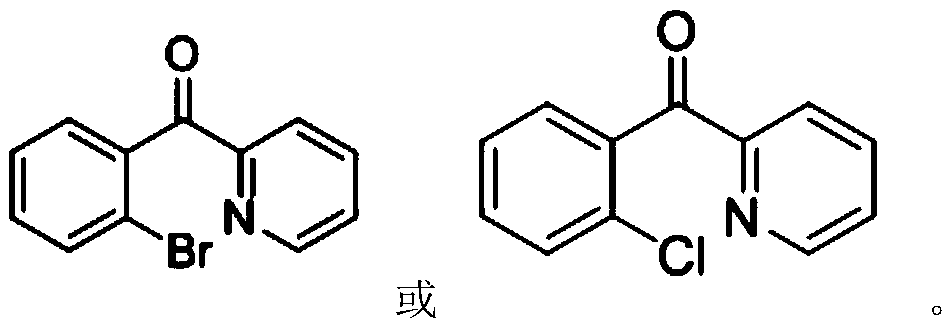
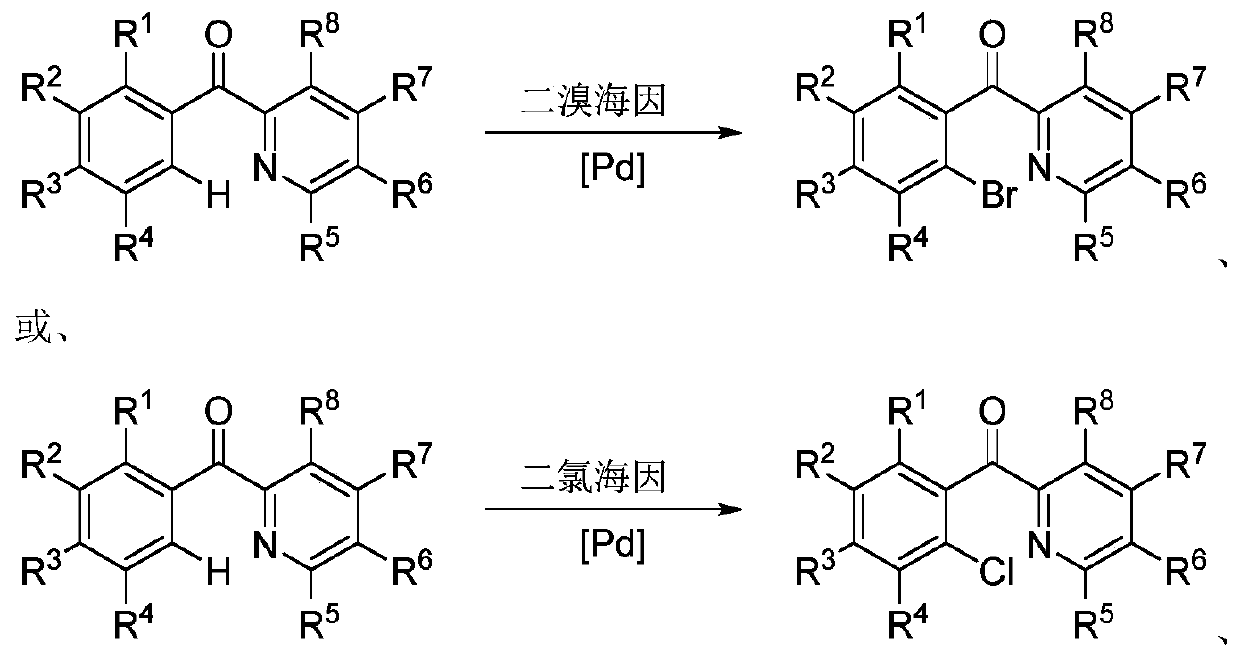
Ortho-halogenated phenylpyridine ketone compound and preparation method thereof
A compound, haloalkyl technology, applied in the field of ortho-halogenated phenylpyridine ketones and their preparation, can solve the problems of large environmental pollution, strong toxicity, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 2-Bromophenylpyridyl Methanone
[0023]
[0024] Phenylpyridinyl ketone (36.6 mg, 0.2 mmol), dibromohydantoin (85.8 mg, 0.3 mmol) and Pd(OAc) 2 (4.5mg, 0.02mmol), added 2mL of chlorobenzene, reacted at 120°C for 12h, purified by thin layer chromatography to obtain 26.5mg of 2-bromophenylpyridyl ketone, yield 50.6%, 1 H NMR (400MHz, CDCl 3 ): δ=8.73(d, J=4.8Hz, 1H), 8.20(d, J=8.0Hz, 1H), 7.98-7.94(dt, J1=7.6Hz, J2=2.0Hz, 1H), 7.68(dd , J1=7.6Hz, J2=1.2Hz, 1H), 7.55-7.50(m, 2H), 7.47(dd, J1=7.6Hz, J2=1.2Hz, 1H), 7.43-7.39(m, 1H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ=195.9, 153.5, 149.4, 140.3, 137.1, 133.1, 131.6, 129.9, 127.1, 127.0, 124.0, 120.1ppm.
Embodiment 2
[0026] Synthesis of 2-Bromophenylpyridyl Methanone
[0027]
[0028] Phenylpyridyl ketone (36.5 mg, 0.2 mmol), dibromohydantoin (85.8 mg, 0.3 mmol) and Pd(TFA) 2 (0.02mmol), add 2mL of chlorobenzene, react at 120°C for 12h, no product is formed.
Embodiment 3
[0030] Synthesis of 2-Bromophenylpyridyl Methanone
[0031]
[0032] Phenylpyridyl ketone (36.6 mg, 0.2 mmol), dibromohydantoin (85.8 mg, 0.3 mmol) and Fe(OAc) 2 (0.02mmol), add 2mL of chlorobenzene, react at 120°C for 12h, no product is formed.
[0033] The catalyst Fe(OAc) 2 Replaced by Cu(OAc) 2 Or under the condition of not adding any catalyst, there is also no product generation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com