Serum-free medium and method for amplifying hematopoietic stem cells
A technology of serum-free medium and hematopoietic stem cells, applied in blood/immune system cells, culture process, tissue culture, etc., to improve the effect of expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
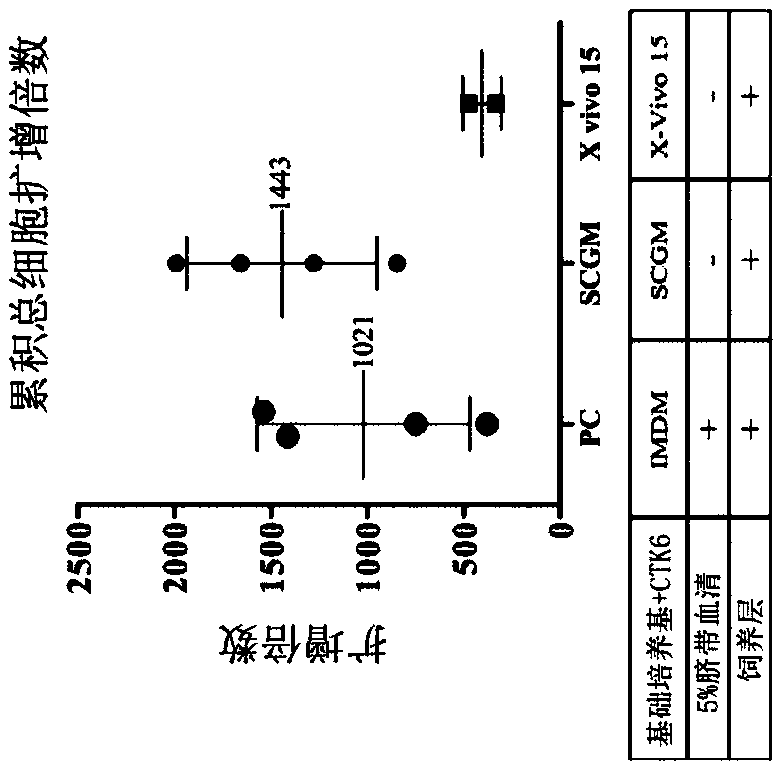
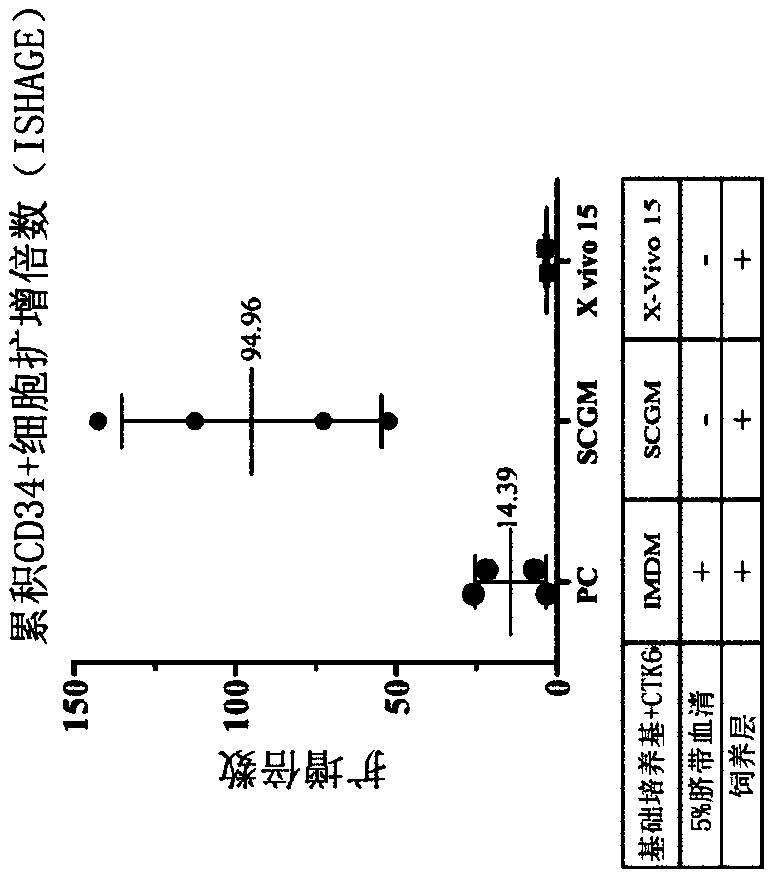
[0093] Experimental example 1: Evaluation of hematopoietic stem cell culture medium
[0094] To evaluate various media for in vitro expansion of hematopoietic stem cells (HSCs), IMDM containing essential cytokines, 5% cord serum (CS) and feeder layers were used as controls. Two media (SCGM and X-VIVO15) were tested in the absence of serum (but in the presence of the same cytokines and feeder layers) to see if they could be used as serum-free media. The detailed experimental steps are as follows.
[0095] On day -1, inoculate umbilical cord mesenchymal stem cells (UC-MSCs) in T-12.5 containers and culture them as feeder cells in complete culture medium (containing 10% human umbilical cord serum and DMEM) . On day 0, CD34+ hematopoietic stem cells were thawed and mixed with the above-mentioned UC-MSC feeder cells at a cell density of 2.5×10 4 Cells / ml were co-cultured for 12 days, and the following different media were used together: (1) Positive control group (PC): containin...
experiment example 2
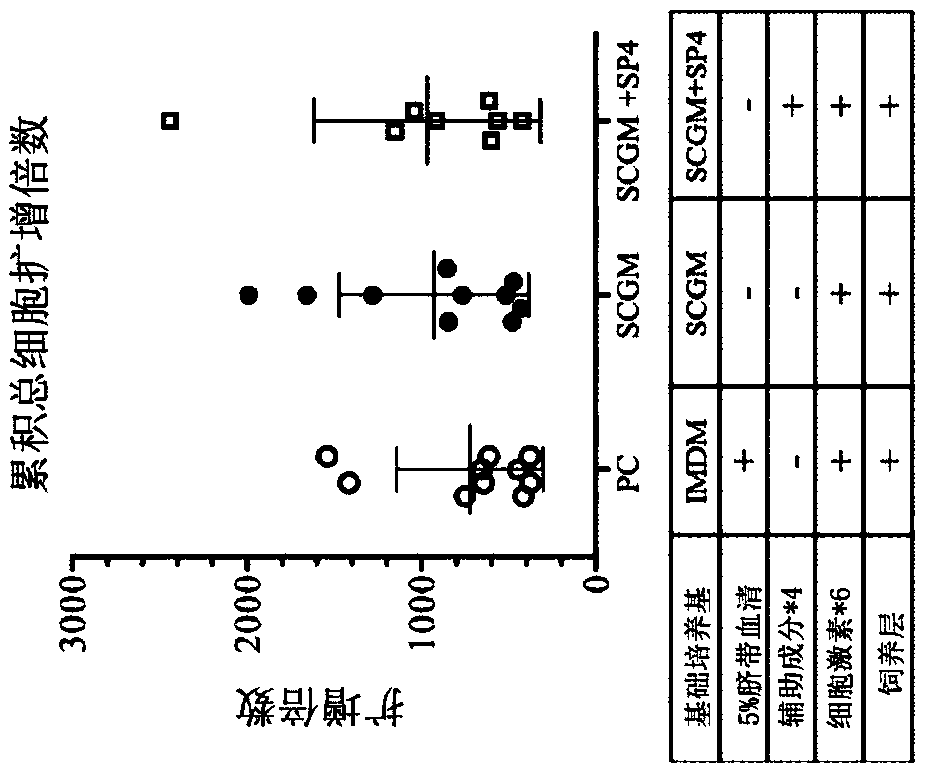
[0099] Experimental example 2: Effect evaluation of auxiliary ingredients
[0100] This experimental example evaluates the effect of adding 4 auxiliary components to the medium containing the feeder layer. The specific experimental steps are as follows.
[0101] On day -1, inoculate umbilical cord mesenchymal stem cells (UC-MSCs) in T-12.5 containers and culture them as feeder cells in complete culture medium (containing 10% human umbilical cord serum and DMEM) . On day 0, CD34+ hematopoietic stem cells were thawed and mixed with the above-mentioned UC-MSC feeder cells at a cell density of 2.5×10 4Cells / ml were co-cultured for 12 days, and the following different media were used together: (1) positive control group (PC): IMDM containing 5% umbilical cord serum, 6 kinds of cytokines and hydrocortisone; (2) SCGM Group: SCGM containing 6 cytokines and hydrocortisone; and, (3) SCGM+SP4 group: SCGM containing 6 cytokines, hydrocortisone and 4 auxiliary components. The six cytok...
experiment example 3
[0103] Experimental example 3: Using serum-free umbilical cord mesenchymal stem cell conditioned medium to replace the feeder layer
[0104] To test potential alternatives to feeder layers, we tested the effect of serum-free umbilical cord mesenchymal stem cell conditioned medium (SF-UCM) derived from umbilical cord mesenchymal stem cells (UC-MSCs). The serum-free umbilical cord mesenchymal stem cell conditioned medium is prepared, for example, by the method mentioned above. The method comprises the steps of: (a) culturing umbilical cord mesenchymal stem cells in a serum-free cell medium (eg, serum-free SCGM); and (b) isolating the conditioned medium. The experimental results of using the umbilical cord mesenchymal stem cell conditioned medium to replace the feeder layer are as follows: Figure 3A and Figure 3B shown.
[0105] exist Figure 3A and Figure 3B Among them, the positive control group (PC) was the above-mentioned IMDM. Figure 3A and Figure 3B The PC and S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com