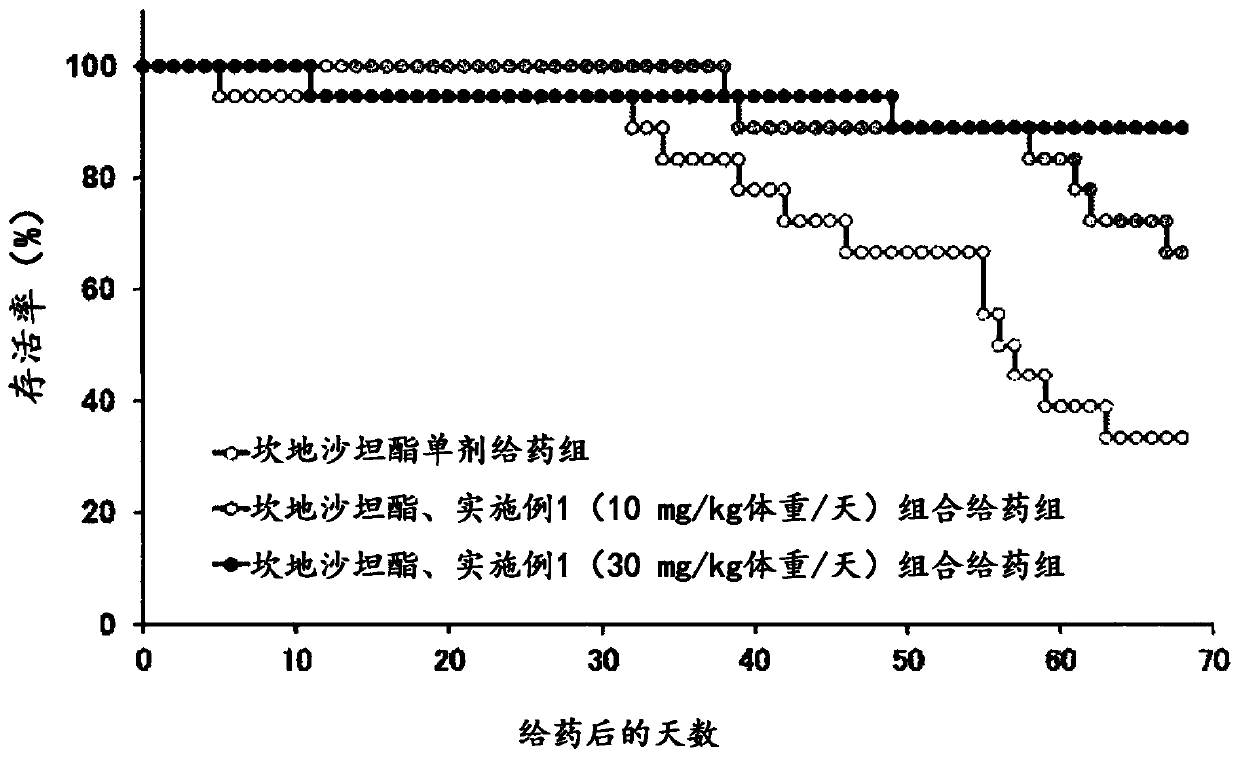
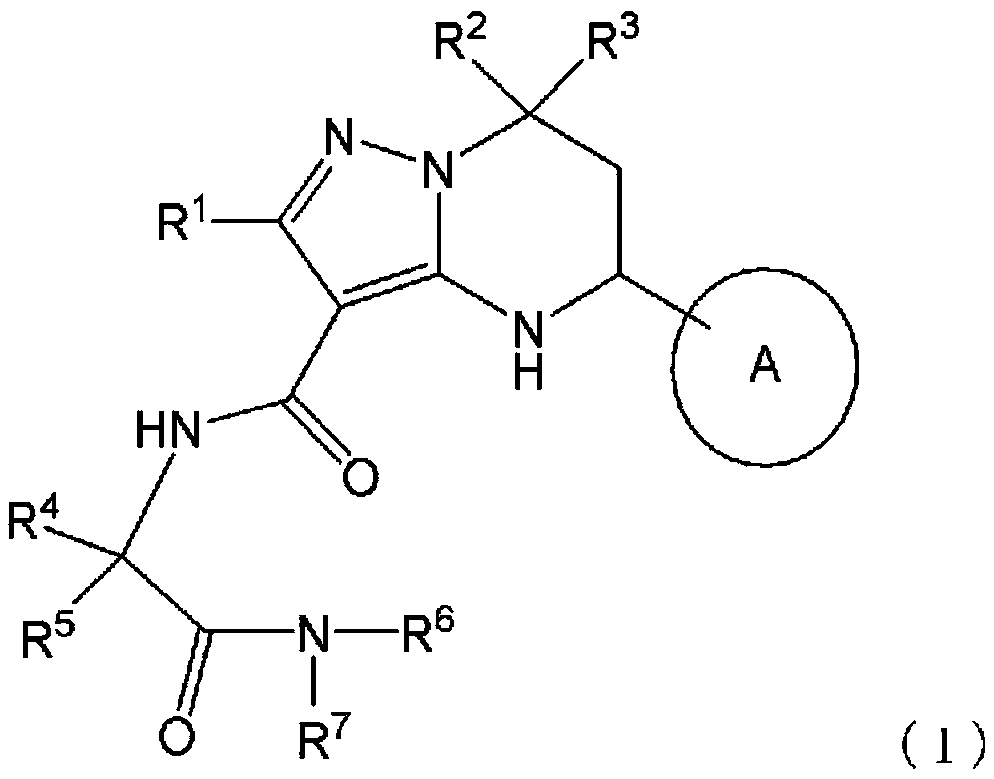
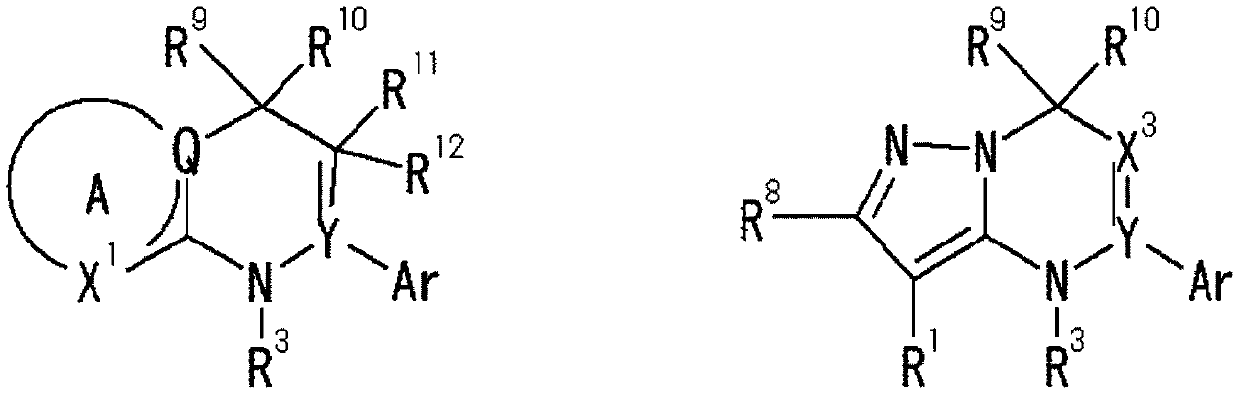
Heterocyclic compounds
A compound and drug technology, applied in drug combination, organic chemistry, cardiovascular system diseases, etc., can solve problems such as deterioration of heart function, insufficient interpretation of parathyroid hormone, and insufficient explanation of improvement in survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0627] (5R)-N-((2R)-1-(((2S)-1-amino-1-oxopropan-2-yl)amino)-2-(4-ethylphenyl)-1-oxo Butan-2-yl)-2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-3-carboxamide
[0628] A) Methyl (2R)-2-amino-2-(4-ethylphenyl)butanoate
[0629] 2-amino-2-(4-ethylphenyl)butanoic acid methyl ester (44.9g) with SFC (CHIRALPAK IC (trade name), 30mmID * 250mmL), mobile phase: carbon dioxide / (methanol / diethylamine= 1000 / 3)=900 / 100 (v / v)) fractionation, and the fraction with shorter retention time was concentrated. Ethyl acetate and water were added to the residue, and the resulting mixture was extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain the title compound (18.2 g).
[0630] Optical purity: >99.9% enantiomeric excess
[0631] The shorter retention time under the following optical analysis conditions
[0632] Column: CHIRALPAK IC (trade name) ...
Embodiment 2
[0646] (5R)-N-(1-(((2S)-1-amino-1-oxopropan-2-yl)amino)-2-(4-ethylphenyl)-1-oxobutan-2 -yl)-7,7-dimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-3-carboxamide
[0647] A) 2-((((5R)-7,7-dimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridin-3-yl) Carbonyl)amino)-2-(4-ethylphenyl)butanoic acid methyl ester
[0648] (5R)-7,7-dimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-3-carboxylic acid (1.655g) was stirred at room temperature , a mixture of HATU (2.78g), DIPEA (0.946g) and DMF (15ml) for 5 minutes. Then a mixture of methyl 2-amino-2-(4-ethylphenyl)butanoate (1.35 g) and DMF (5 ml) was added, and the mixture was stirred at 80° C. overnight. Water was added to the mixture, and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane / ethyl acetate) to obtain the tit...
Embodiment 3
[0656] (5R)-N-((2S)-1-(((2S)-1-amino-1-oxopropan-2-yl)amino)-2-(4-ethylphenyl)-1-oxo Butan-2-yl)-2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-3-carboxamide
[0657] A) 2-(4-ethylphenyl)-2-((((5R)-2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo [1,5-a]pyridin-3-yl)carbonyl)amino)butanoic acid methyl ester
[0658] (5R)-2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-3-carboxylic acid (516mg ), HATU (825 mg), a mixture of DIPEA (280 mg) and DMA (5 ml) for 5 minutes. Then, a mixture of methyl 2-amino-2-(4-ethylphenyl)butanoate (400 mg) and DMA (2 ml) was added, and the resulting mixture was stirred at 80° C. for 2 days. Water was added to the mixture, and extracted with ethyl acetate. The organic layer was separated, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane / ethyl acetate) to obtain the title com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com