Method for synthesizing AMG837
A technology for compounds and intermediates, applied in the field of synthetic compounds, can solve the problems of expensive metal catalysts, long routes, low yields, etc., and achieve the effects of shortening the synthesis route, good repeatability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
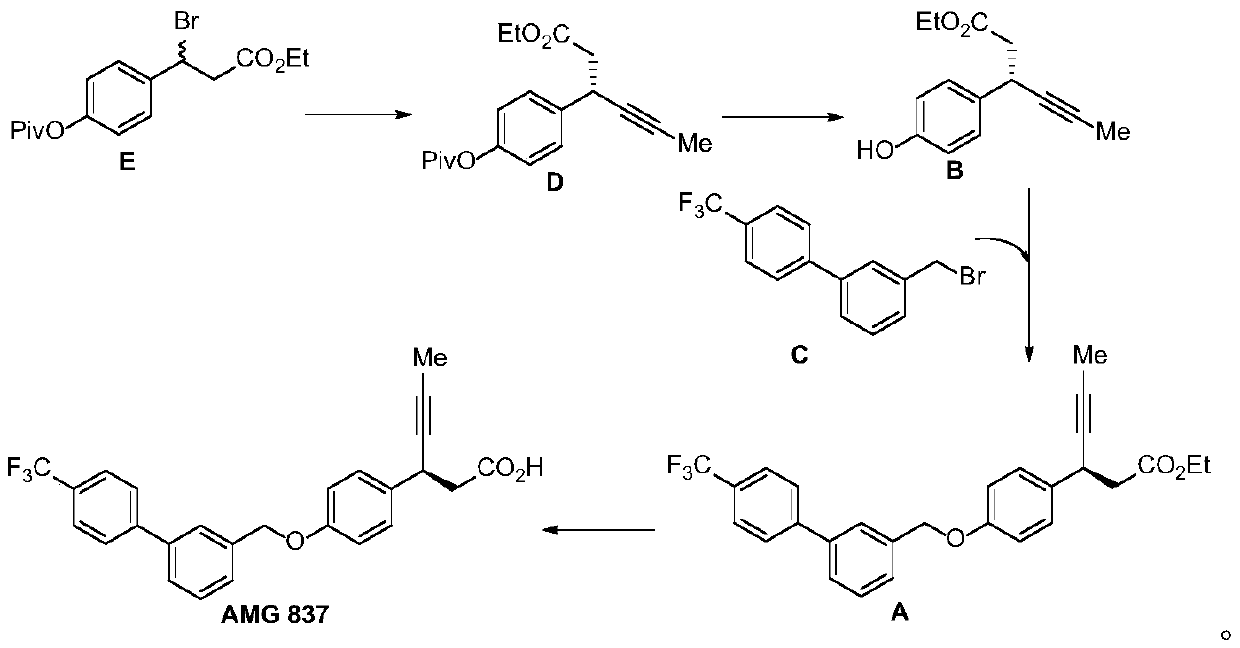
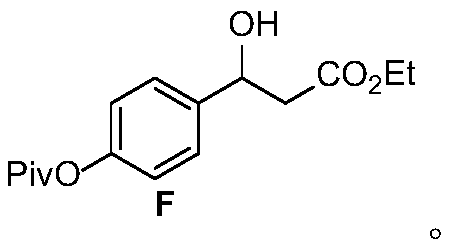
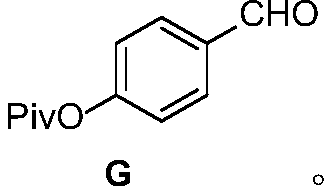
[0053] The synthetic route of AMG837 is as follows
[0054]
[0055] The specific steps are:
[0056] (1) Synthesis of Intermediate C
[0057] According to the method reported in the literature: B.M.Trost, J.T.Masters, B.R.Taft, J.-P.Lumb, Asymmetric synthesis of chiralβ-alkynyl carbonyl and sulfonyl derivatives viasequential palladium and copper catalyst. Chem.Sci.7,6217-6231(2016) .)
[0058] Pd(dppf)Cl was charged to an oven-dried 100 mL round bottom flask equipped with a stir bar 2 (365.9 mg, 0.50 mmol, 5 mol%) and 3-hydroxymethylphenylboronic acid (1.90 g, 12.5 mmol, 1.25 equiv). The flask was sealed with a septum and the gas was displaced with Ar, then DME (20 mL) was added. Add Na under argon flow 2 CO 3 (2.12 g, 20.0 mmol, 2.0 equiv), then 4-bromobenzotrifluoride (1.40 mL, 10.0 mmol, 1.0 equiv) was added via syringe. The vessel was heated to 80°C, then sealed with a yellow plastic cap, and the reaction mixture was stirred for 18 hours. The container was cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com